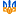|
Начало раздела Производственные, любительские Радиолюбительские Авиамодельные, ракетомодельные Полезные, занимательные |
Хитрости мастеру Электроника Физика Технологии Изобретения |
Тайны космоса Тайны Земли Тайны Океана Хитрости Карта раздела |
|
| Использование материалов сайта разрешается при условии ссылки (для сайтов - гиперссылки) | |||
ПРИСПОСОБЛЕНИЯ А также ТЕХНОЛОГИЯ ХРОМИРОВАНИЯ ИЗДЕЛИЙ
![]()
Смотри так же: |
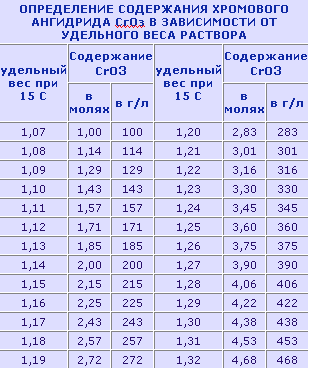
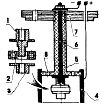
Хромирование, одно из практичных нужных двигателистам покрытий, относится к наиболее трудоемким процессам гальванотехники. Оно требует особой тщательности также соблюдения чистоты как будто при подготовке электролита, так также самих веществ, входящих в его состав. Вода используется дистиллированная либо (лишь в крайнем случае!) основательно прокипяченная. Изготовление ванны Занятия модельной гальванотехникой начните с изготовления ванны, Предварительно всего подберите кастрюлю на 10 л также трехлитровую стеклянную банку. Емкости меньшего размера лучше никак не применять - это может усложнить регулировку параметров процесса, разумеется также при приведенных величинах емкости ванны хватает лишь только для хромирования 6-8 гильз цилиндров. Склеив из 1-1,5 мм фанеры корпус, соберите ванну согласно приведенному рисунку также закройте все фанерным кольцом. Труд над ванной заканчивается вытачиванием крышки кастрюли также монтажом на ней ТЭНов также контактного градусника. Нынче - электрооборудование. Для кормления ванны можноиспользовать всякий родник неизменного тока с подключенным на выходе электролитическим конденсатором 80000 мкф X 25 В. Провода кормления должны владеть сечение никак не меньше 2,5 мм2. Регулятором силы тока, заменяющим регулятор напряжения, может прислуживать секционный реостат. Он включается подряд с гальванической ванной также состоит из параллельных, включаемых однополюсными рубильниками секций. Каждая последующая владеет противодействие вдвое больше предыдущей. Количество таких секции 7-8. На передней панели блока литания установите две розетки на 15 А, одну - нормальной полярности, другую-обратной. Это позволит спешно провести анодную отделку детали также перейти на хромирование бесхитростным переставлением вилки. Розетки с тремя выходами, чтобы никак не ошибиться в полярности (подключаются, несомненно, только пара гнезда). Для поддержания постоянной температуры электролита ванна снабжается контактным градусником. Напрямую управлять трудом ТЭНов он никак не может из-за внушительных токов, поэтому потребуется собрать несложное устройство, схема которого приведена на рисунках.
Детали терморегулятора: транзисторы VT1 - (МП 13 - МП16, МП39-МП42); VT2213-217 (П213-П217) с любыми буквенными обозначениями; резисторы - (МЛТ-0,25); диод - (Д226, Д202-Д205); реле - (ТКЕ 52 ПОДГ либо ОКН паспорт РФ4.530.810). Наладка терморегулятора: если при закорачивании точек 1-2 репе никак не срабатывает, соединяют эмиттер также коллектор VT1. Включение реле указывает на неисправность либо маленький коэффициент усиления VT1. В противном случае неисправен транзистор VT2 либо он владеет недостаточный коэффициент усиления. Собрав также наладив уклад ванны, можноприступать к подготовке электролита. Для этого необходимо: Последняя операция нужна для накопления небольшого числа монов Сг3 (2-4 г./л), пребывание которых дружественно сказывается на процессе осаждения хрома. Состав электролитов Хромовый ангидрид-250 г/л либо 150 г/л Режимы хромирования Процесс хромирования в сильной степени зависит от температуры электролита также плотности тока. Оба фактора действуют на наружный вид также свойства покрытия, но похоже на выход хрома по току. Необходимо не забывать, что с повышением температуры выход по току снижается; с повышением плотности тока выход по току возрастает; при более низких температурах также постоянной плотности тока получаются серые покрытия, но при повышенных - молочные. Практичным маршрутом найден оптимальный режим хромирования: плотность тока 50-60 А/дм2 при температуре электролита 52° - 55° ±1°. Чтобы быть уверенным в работоспособности электролита, в приготовленной ванне можнопокрыть несколько деталей, подобных по форме также размерам рабочим образцам. Подобрав режим также узнав выход по току бесхитростным замером размеров вплоть до также позже хромирования, можноприступать к покрытию гильз. По предложенной методике накладывают хром на стальные, бронзовые также латунные детали. Подготовка их заключается в промывке поверхностей, подлежащих хромированию, бензином также затем мылом (с помощью зубной щетки) в горячей воде, зарядке в оправку также размещении в ванне. После погружения в электролит нужно подождать 3-5 с также затем включить рабочий ток. Задержка нужна для того, чтобы деталь прогрелась. Сразу проистекает активирование поверхности деталей из латуни также меди, так как будто данные металлы хорошо травятся в электролите. Но больше 5 с медлить никак не следует - в составе этих металлов есть цинк, пребывание которого в электролите недопустимо.
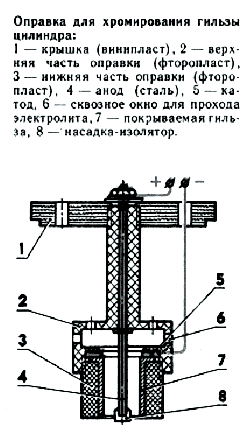
Хромирование алюминиевых сплавов На процессах нанесения хрома на алюминиевые сплавы нужно остановиться особо. Выполнение таких покрытий прктически всегда сопряжено с рядом трудностей. Предварительно всего это необходимость предварительного нанесения промежуточного слоя. Сплавы алюминия, содержащие внушительное число кремния (до 30%, сплавы марок АК12, АЛ25, АЛ26, САС-1), можнохромировать следующим образом: Другое занятие, если необходимо покрыть хромом сплав АК4-1. Его удается отхромировать только с помощью промежуточного слоя. К таким методам относятся: цинкатная обработка; по подслою никеля; чрез соль никеля; чрез анодную отделку детали в растворе фосфорной кислоты. Во всех случаях детали подготавливают следующим образом: Для травления используется раствор едкого натра (50 г/л), пора отделки 10-30 с при температуре раствора 70-80°. Для травления сплавов алюминия, содержащих кремний также марганец, лучше использовать такой раствор, в весовых частях: Промежуточные покрытия Цинкование Если цинковое покрытие легло неравномерно, деталь опускают в стравливающий 50-процентный раствор азотной кислоты на 1-5 с также позже промывки повторяют Цинкование. Для магнийсодержащих сплавов алюминия двойное Цинкование обязательно. Нанеся другой слой цинка, деталь промывают, заряжают в оправку также под током (без подачи напряжения цинк успевает частично раствориться в электролите, загрязняя его) устанавливают в ванне. Предварительно оправка с деталью погружается в стакан с водой, нагретой вплоть до температуры 60°. Процесс хромирования обычный. Никелирование (Химическое) Последняя операция - в растворе следующего состава: сернокислый никель 30 г/л, гипофосфит натрия 10-12 г/л, уксуснокислый натрий 10-12 г/л, гликоколь-30 г/л. Составляется он сначала без гипофосфи-та, какой вводится пред никелированием (с гипофосфитом раствор продолжительно никак не хранится). Температура раствора при никелировании 96-98°. Можно использовать раствор также без гликоколя, в то время температура должна быть снижена до 90°. За 30 мин на деталь осаждается слой никеля толщиной от 0,1 вплоть до 0,05 мм. Посуда для работ - только стеклянная либо фарфоровая, так как будто никель осаждается на все металлы восьмой группы периодической таблицы. Хорошо поддаются никелированию латунь, бронза также другие медные сплавы. После осаждения никеля проводится термообработка для улучшения сцепления с основным металлом (200-250°, выдержка 1-1,5 ч). Затем деталь монтируется на оправке для хромирования также опускается на 15- 40 с в раствор 15% серной кислоты, в каком месте обрабатывается обратным током из расчета 0,5-1,5 А/дм2. Происходит активирование никеля, удаляется окисная пленка, также покрытие приобретает серый цвет. Кислота должна применяться только химически чистая (в самом крайнем случае аккумуляторная). Другим образом никель приобретает черный краска, также хром на такую поверхность ни в жизнь никак не ляжет. После этого оправку с деталью загружают в ванну хромирования. Вначале позволяют ток в пара раза больший, затем в течение 10-12 мин его уменьшают вплоть до рабочего. Дефекты химического никелирования: Удаление никеля с алюминиевых сплавов можнопроизводить в растворе азотной кислоты. другой раз в процессе никелирования проистекает саморазряд - выпадение порошкообразного никеля. В этом случае раствор выливают, но посуду обрабатывают раствором азотной кислоты для удаления с ее поверхности никеля, какой станет препятствовать осаждению на детали. Хотелось бы отметить, что никель-фосфор самолично по себе обладает очень интересными свойствами, никак не присущими хромовым покрытиям. Это равномерность слоя на поверхности деталей (после осаждения доводки никак не требуется); высокая твердость позже термообработки (режим 400° в течение часа отчуждает твердость покрытия HV 850-950 также больше); невысокий коэффициент трения по сравнению с хромом; весьма незначительное расширение; высокий предел прочности при растяжении. Никель-фосфор без дальнейшего нанесения хрома может использоваться никак не только как будто промежуточное покрытие на гильзах, но также как будто рабочее, снижающее трение также износ, для золотников также поршневых пальцев. После пары лет активной эксплуатации двигателя с деталями подобной отделки на них отсутствовала явная выработка, характерная для стальных каленых поверхностей. Нанесение хрома чрез соль никеля Нанесение хрома чрез анодную обработку После анодной отделки следует провести кратковременную катодную отделку в щелочной ванне, которая частично снимает оксидный слой. Как показали исследования, в процессе анодной отделки алюминиевых сплавов в фосфорной кислоте на деталях образуется шероховатая поверхность, которая способствует прочному сцеплению наносимого впоследствии покрытия. Приспособления, оправки Хромирование гильзы
Анод - стальная шпилька; с одного ее конца на длине 50-60 мм наплавляется свинец с сурьмой (7-8%). Свинец протачивается по наружному диаметру до 6 мм (для гильз рабочим 0,15 мм). С другой стороны шпильки нарезается резьба для фиксации провода. Катодом служит кольцо с внутренним диаметром, на 0,5 мм превышающим внутренний размер гильзы. В него вчеканивается отрезок изолированного провода. Медные также латунные проводники лучше никак не использовать - электролит растворяет их, иконтакт может быть нарушен. Перед монтажом оправки в ванне полезно проверить прочность контактов тестером. Хромирование стальных деталей Расчетный ток определяется перемножением площади хромируемой поверхности на ток процесса. Для стали последняя размер-50 А/дм2. При хромировании, например, посадочного помещения под коренной подшипник на коленвале двигателя КМД-2,5 расчетный ток станет равен 0,03 дм2 x 50 А/дм2 x 1,5 А. Для хромирования пальца кривошипа понадобится новая оправка. Как также при отделке коленвала, все открытые участки поверхности закрываются клеем "АГО". Анод вытачивается из стали с последующей заливкой свинцом также расточкой отверстия под палец. Применение стальной детали объясняется необходимостью обеспечить безопасный контакт - в свинце резьбовые соединения ненадежные. Расчеты токов схожи. Труд проводится в оправке вала с помощью специальной насадки. Почти что ничем никак не отличается хромирование подшипников. Единственное - для предохранения внутренней элементы детали ее заполняют солидолом либо другой консистентной смазкой, которая позже нанесения покрытия вымывается бензином.
Оправка для хромирования внешней обоймы шарикоподшипника: Дефекты хромирования также их повода
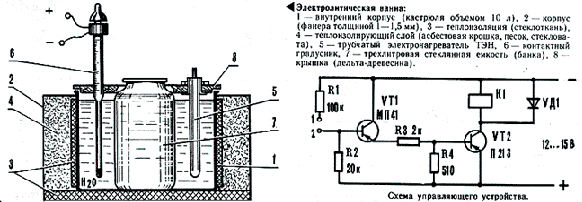
Концентрация хромового ангидрида в электролите контролируется с помощью ареометра. Концентрацию же серной кислоты удается определять лишь, к сожалению, косвенно, по качеству покрытия. В процессе хромирования движется испарение электролита. В этих случаях доливают воду вплоть до нужного уровня. Делается это без установки деталей - возможно изменение температуры электролита. После хромирования все изделия подвергают термообработке в течение 2-3 ч для удаления водорода, при температуре 150-170°. Все труда ведутся под вытяжным приспособлением, в резиновых перчатках также в очках. По материалам журнала "Моделист-Конструктор". Исток в №5 за 1989г. Created/Updated: 25.05.2018 stop war in Ukraine stand with Ukraine |