|
Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
ELECTROTYPE
APPLICATION OF METAL COATINGS FOR JEWELERY DECORATIONS
![]()
See also: |
Metallization of objects with electroforming is an activity that is available at home to anyone who wants to master this matter. Lead electroforming labor in the vessels-baths, usually having a rectangular (also can be another) capacity, determined by the capacity of those things that are to be reproduced. It is also glass, also ceramic (glazed) vessels, also plastic (in particular, boxes from batteries or welded containers of sheet wine), as well as wooden boxes covered with bitumen. The galvanoplastic method applies metal to a variety of objects. For example, turn ordinary lace into metal (they are decorated with rims for pictures or boxes, they are made of bracelets, other filigree delicacies). To carry out the electroforming coating, we need a spring of a constant current of low voltage ( 3- bV), for which quite powerful selenium also other rectifiers will go. The most accessible rectifiers are designed for charging car batteries (current up to 7 A , 6 V voltage), or dry elements (if labor is small). Adjust the current strength, the density of which in the process of labor is 1-2 A / dm2 , most often sliders or water rheostats.
The shape (cathode) is also strengthened in a copper bath (anode) in a bath on suspensions, a copper electrode on a copper or brass hook so that the hole in the electrode also does not touch the electrolyte in any way (otherwise the metal will become corroded). Suspend the form on copper or brass wire at a distance of 15-20 cm from the electrode. As an anode for a copper electroformed bath, a copper plate 3-4 mm thick is also used.
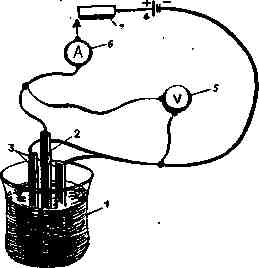
Fig. 1 Scheme of electrotype installation:
1 - a bath, 2 - an anode, 3 - cathode-forms for building up copper,
4 - source of constant current, 5 - voltmeter, 6 - ammeter, 7 - rheostat
A galvanoplastic method can produce a variety of sculptures or metal jewelry. And forms for the deposition of metal are prepared from gypsum, wax, paraffin, plastic, plasticine, especially the "Vixint" sealant is particularly suitable in this respect. Forms of wax or gypsum pre-operate electrically conductive, covering a layer of material of a certain composition (graphite or bronze powder). This layer is also connected to the negative pole.
The electrolyte is prepared on the basis of copper sulfate with the addition of sulfuric acid, which increases the electrical conductivity of the mass. For 1 liter of water will need 150-180 g of copper sulfate (copper sulfate). Dissolve better in hot water. After the cooling solution is filled (to room temperature), the electrolyte is filtered through the tissue. Then it is carefully poured already with sulfuric acid (slowly, with a thin stream in order to avoid the rapid heating of the electrolyte, its spraying, which sometimes leads to severe burns). The essence of sulfuric acid in copper sulfate baths is maintained in the range of 35-40 g / l (its density is 1.84 g / cm3 ). The solubility of copper sulfate decreases markedly with the increase in the number of acids. In a solution with an increased occurrence of copper sulfate, it crystallizes on the walls of the bath and also, worse, on the anode. This makes the electrolysis process difficult. Excess sulfuric acid also causes fragile and poor-quality deposits of copper due to hydrogen, which is intensively released at the cathode, especially if we have a job with increased current densities. And an inadequate concentration of sulfuric acid leads to the formation of a loose and porous copper deposit, which is of no use at all. Sometimes the quality of copper is increased by applying additives. For example, alcohol ( 8-10 g / l ). The presence of alcohol greatly improves the quality of copper. But to all its own norm - the excess of alcohol acts copper brittle. The ingress of organic substances into the electrolyte (glue, some types of rubber, etc.) adversely affects its functioning. It is possible to remove such impurities by oxidizing the preheated electrolyte with potassium permanganate ( 2-3 g / l ) or eliminating by means of finely crushed activated carbon (also 2-3 g / l ), after which it is filtered.
In ordinary electroforming electrolytes, a room temperature ( 18-20 ° C ) is maintained. It can rise to 25-28 degrees. As a result of the release of heat during the passage of the electric current through the electrolyte, the electrolyte is filtered as if it is possible more often, removing sediment from the baths - sludge in the form of powdered copper, graphite is also a speck of dust.
The current density is very important. The higher it is the more the anodes dissolve more intensively, the more the slime accumulates in the bath (especially when low-grade anode copper is used). Sludge, as if the situation, settles to the bottom. But its lighter particles in the suspended state due to convection move to the cathode also cause clogging of the electroformed copper. In contact with the copper deposited on the cathode, the sludge is included in the metal, forming roughness also cones that interfere with the further deposition of the metal. Graphite, used as an electrically conductive layer covering the mold, also contaminates the electrolyte, splashes into the metal, and also contributes to the roughness of the surface.
And now, after general information, - directly to the topic of this section. All those interested in obtaining a copper sculpture with the electroforming technique, refer to NV Odnoralov's article on this problem published in the Do-It-Yourself series ( 1990, No. 2 ). We will dwell in detail on the build-up of metal on the model.
On the wax-like plasticine models - all in all. This method of building up is used, if in no way requires a special precision of reproducing the parts, and they can be subjected to mechanical finishing (cutting, filing, chasing, etc.). First of all thin-walled artistic products without joints. Wax models made of ozocerite or a composition containing, in addition to ozocerite, 50% w / w paraffin (or stearin), also having a sufficiently low melting point, also by slight shrinkage, only after hardening, by substantial hardness. Before pouring the composition into the gypsum form, the contacting conductors are laid in the form of hooks or knots, not forgetting deeply profiled rooms of the future model, which are protrusions in gypsum form. Conductors data after pouring protrude over the model with bent ends. In the form of stacked similarly brass or copper rod, the servant of the frame is also a contacting suspension that connects to the bar of the bath. When the wax composition solidifies, a copy of the model is taken out of the gypsum mold, the seams arising during the casting of the wax pattern are removed.
When increasing the details on the top, decisive is the speed with which they are tightened with metal in an electroformed bath. It depends on the quality of the applied electroconductive layer also from the error-free location of the contacting conductors. After the deposition of a layer of metal of the appropriate thickness ( 1.5-2 mm ), which does not distort the relief in any way (but sufficient for labor by the coins), it is treated with ordinary files or with ribbons. Then they strike through. Wax composition is heated.
Manufacturing of metal molds for casting sculptures (products) from plastics. It is conducted by the method of contact copying from models of sculpture. To obtain molds for reproducing sculptures from foundry plastics, models are pre-made in the same way as if they were also being built on top. In a piece of gypsum moistened form, a special wax composition is poured. For example, such a composition: 700 g of ozocerite, 200 - paraffin, 100 - rosin. The finest graphite electroconductive layer is applied to the obtained wax models (rubbed with sifted graphite dust). Then on the model install the conductors also under the current immersed in the electrolyte.
With the appearance of a metal layer of the desired thickness, the wax from the metal forms is melted, heating them over the steam. Rinse them with gasoline, acetone and other solvents, degrease with hot alkali. Rinse with hot water. In these molds, having differences of high accuracy, lightness is also strength, plastic resin is poured. For example, epoxy, resins, neoleucrite, with excellent casting. After condensation of the resin (solidification-filled plastic is produced in machine oil, poured into an iron vessel, the mold is set in it, and the oil is heated to 60-70 degrees ), the forms are removed from the finished sculptures, dissolving them in the same copper sulphate electrolyte, Forms. They are hung on the anode. At the same time, they are building up a new view of the wax models completed on the cathode.
Graphing. To the greatest extent, the properties of creating an electrically conductive layer give a response to flake graphite. But you can use any sort of this material. It is necessary that the graphite, which rubs the mold, be clean, has no extraneous impurities, was not large-scaly or matt (earthy, soot-colored). Before use, it is processed. The usual petty scaly is ground in a porcelain mill (with water) or ground in a mortar. The smallest, colloidal, in a colloid mill. Remove iron oxides: knead with water until the creamy mass, add hydrochloric acid - in a day the graphite is deposited on the bottom of the vessel. The water is drained. Graphite is repeatedly rinsed with water - until the acid is completely removed. Dry them. Razirayut spatula. Sift through a thin metal or silk sieve (with a minimum of 400 pcs / cm .) For imposing also very accurate copies, the finest graphite will be needed.The production of copies of an impressive size is more suitable for large-scale ones - it has an increased electrical conductivity.
It must be borne in mind that graphite has an appreciable electrical resistivity. And from inaccurate rubbing them ohmic resistance can increase. Therefore, it is applied with a dense layer. On the forms with a delicate relief - a brush of soft, but not very long hair (this is to use it end). On the brush put on a rubber tube, protecting the form from the feasible contact with the metal mandrel brush. Usually watercolor column brushes from № 8 up to № 14 ; Rarer - more rigid, used in oil painting; Use similar cotton swabs (mainly for rubbing gypsum forms).
Gypsum, impregnated with wax, as well as wax forms, is best graphitized in a still not completely cooled state (the adhesion of particles of one substance to another is high). In this case, graphitize in 2 steps. Another warm form is gently pripudrivayut cotton swab, applying graphite in excess. And later the cooling form is graphitized definitively. On a soft wax composition made of soft plasticine, use soft squirrel brushes or cotton swabs. The walls of the same cavity of gypsum form, on the contrary, it is even better to graphite with a sufficiently rigid brush, paying attention to narrow or deep details of the relief. Graphing with a cotton swab, one should often look at its working surface - it can also wax to damage the relief.
Paraffin forms are more difficult to graphitize (the material applied to them does not adhere well to their surface). Usually you need a long graph. The forms are treated with a brush, but the tampon does not follow (the paraffin is also fragile from the rubbing, it tends to peel off).
Plasticine molds, reliefs are also three-dimensional figures, before applying graphite on them, they are covered with shellac • varnish or nitro-lacquer, thereby creating a film that protects the surface layer of plasticine from damages during graphitization also from erosion by electrolyte. Plasticine bas-reliefs are made on a plastic or glass plate, creating a flat background. The voluminous sculptures of plasticine, on which metal is built, operate on aluminum frames. In the case when the support of the carcass comes out, it is covered with paraffin or wax. But the protruding part of the carcass is left until the end of the galvanoplastic process (it is convenient to use the carcass to suspend the sculpture in the bath), And only after its termination this share is cut off with a hacksaw, densely covering the incision with clay. It is covered with an electrically conductive layer also builds up the metal in the electrolyte.
On glass, plastic and other materials, on which the metal is built up mainly for decorative reasons, graphite is applied as follows. The material to be graphitized is first covered with a thin rubber or wax layer - apply a spray or brush with a 0.2-0.3 solution of one or the other. And after that, put graphite with a soft brush.
Wood, lace, paper, as well as other hygroscopic materials, up to metal extrusion, are impregnated with paraffin or wax, then graphitized.
It is necessary to additionally additionally subform the forms, partially already expanded by metal. And all because in the process of electroforming metal to nonmetallic forms, the surface fraction is sometimes not tightened by the build-up elements due to insufficiently dense graphite deposition, insufficient wetting of the whole form by the electrolyte, and the separation of bubbles on it also for other reasons. Do not pay attention to this, and you will further build up - there are significant pores in the thickness of the metal. To prevent this from happening, the molds are removed from the electrolyte in advance, washed in a running water bath or in a weak stream of water, the unattached rooms of the mold are also dried by a stream of cold atmosphere or filter paper. The data of the room is then labeled with a soft brush, preferably with an edge (it is suitable not only for the surface of the mold, but also for the walls of small holes). It is impossible to sign cotton wadding with gauze tampons either - the fibers adhere to the mold, and the build-up metal becomes roughened.
The graphite-coated forms are blown, removing the excessive, with it not connected at all; Especially those with a complex deep relief.
Bronzing , i.e., means of bringing up an electrically conductive layer by applying a bronze powder, is less common than graphing. The fact is that the bronze powder loosely adheres to the materials, from which the molds are usually made. But still. Brush the powder with a brush. Moisten the surface with a 15-25% solution of alcohol. Immediately, the alcohol is removed and applied to the form heated to 30-35 deg. A solution consisting of 6 g of silver nitrate and 50 g of sodium thiosulfite diluted in 1 liter of water. Once the color of the mold surface changes, the solution is drained. Pour fresh. By purchasing a gray color, which is already unchanged, the last portion of the solution is drained. The form is thoroughly washed with water.
Silvering is also not used very often. Increase the wettability of the mold is achieved by finishing it in no less than 1-2 minutes with alcohol (after this) for 2-5 minutes with a solution of the following composition: 5 g of tin chloride, 40 ml of hydrochloric acid, 1 liter of distilled water. By the way, tin chloride is also immediately a catalyst, also a reducing agent for silver. After washing the form with distilled water, proceed to silvering. Prepare 2 solutions: 1st - 40 g of silver nitrate, 1000 g of distilled water; 2 nd - 7 g pyrogallol, 4 g citric acid. Then the 1 st also 2-nd solutions are mixed in a ratio of 1: 5 by weight and poured onto the mold. After drilling the solution, it is drained. The form is washed with distilled water and the silvering operation is repeated with the same brown solution. Having finished silvering, the form is dried.
The mold is also coated with silver sulphide. Treated with 5-8% tin chloride, the form is poured (or brushed) with a solution: 10 g of silver nitrate, 25 ml of ammonia ( 25% ), 30 ml of ethyl alcohol, 20 ml of distilled water. The wetted form is dried and placed in a chamber with hydrogen sulphide or blown in a fume hood. To get two hydrogen sulfide, poured into the porcelain cup pieces of iron sulphide are also doused with hydrochloric acid. Blowing the shape of the atomizer, fix it so that the branch pipe is at some interval from the liquid, only on the bottom of the bubble ammonium sulfate is poured. Under the action of hydrogen sulfide on the deposited layer of ammonia silver, a thin film of silver sulfide is formed, which has a sufficiently high electrical conductivity.
A means of obtaining a film of silver sulphide on the layer of shellac varnish is quite rare. The mold is covered with a thin layer of lacquer, and then the drying is immersed in the solution (or the final one is applied with a brush) consisting of silver nitrate and also alcohol, taken in the ratio 2: 3 by weight. The wet form is placed in a chamber with hydrogen sulphide or blown with a jet. Alcohol solution of silver nitrate softens the surface layer of shellac, why it is better kept on the surface of the mold.
Copper can metallize the surface in this way. On the previously graphitized form, 50% alcohol solution is applied (this improves its wettability). Then - 20% solution of copper sulphate. Add the last 15% solution of rectificate alcohol. Another wet surface of the mold is sprinkled with very fine iron filings, which are mixed with a soft brush. The process is repeated 2-3 times. Prior to copper plating, the product is degreased by contact precipitation from an ammonia solution of copper glycerate. Slightly reduces the smoothness of the surface (eg, glass is treated with a skin or etched with hydrofluoric acid) to improve adhesion to the metal being deposited. Products made of plastic are rubbed with tooth powder or magnesium oxide, mixed in a 10-15% solution of potassium carbonate or other alkali. Porcelain or glass products are immersed for 1-2 minutes in a powerless solution of hydrofluoric acid. After preparation, the object is thoroughly washed with a jet of water. Dip in a 1% solution of silver nitrate for 5 minutes and also dry at 40-50 degrees. .
The product lingers, lowering it for 10-20 minutes in a warmed to 25-30 degrees. A composition comprising 1.1 l of the so-called copper solution, 400 ml of a 3% sodium hydroxide solution, 200 ml of a reducing agent and 800 ml of formalin. "Copper solution" - the following composition: 1 L of copper sulfate ( 3% solution), 20 ml of concentrated ammonia, 70-80 ml of glycerol. Reducing agent: 100 g of sugar are dissolved by heating in 250 ml of water, 0.5 ml of concentrated nitric acid is added. Warm up the solution until it is amber. Then dilute it with water up to a capacity of 1250 ml. The copper-coated articles of the mold are thoroughly washed with water and also loaded into an electrolytic bath to build up the metal.
Electrolytic build-up is the main theme of this subsection. We will calculate that the molds prepared for it are already equipped with conductors that have a contact with the electroconductive layer also with a suspension for attachment to cathode rods, that is, they are charged. If the density of the mold materials is less than that of the electrolyte, it is supplied with weights that also store it under the upper level of the electrolyte.
Charge forms. Conductors operate from a very soft, as if annealed also etched copper or brass wire 0,15-0,2 mm or 0,3-0,5 mm . Thin wires are also used for small forms. Potolshche - on large (the use of conductors of a larger diameter allows to increase the current density). In forms taken from reliefs or volumetric sculpture, there are holes for contacting suspensions or conductors also hanging loads. These holes in wax forms are usually calcined, at a time when wax is still soft enough. In gypsum, it is drilled manually until the forms are impregnated with a wax composition. Have openings in the non-working edges of the mold: their diameter is such that they can introduce contact wires or suspensions, the cross-sectional area of which ensures that there is no heating, taking into account the maximum operating current density. For flat forms, the cargo openings are on the opposite side of the suspension holes. The number of such holes is selected on the basis of the need to balance the shapes in the bath. The contacting conductors are laid at a distance of 5-10 mm from the boundaries of the finished product. This alienates the possibility of easily separating the metal debris when decorating the finished bas-relief. To place the conductors away from the boundaries of the form is proud because they are covered with the thickest layer of metal, making it difficult to remove the flare. In bulk as well as lumpy forms, the conductors are firstly reinforced at the end. The conductors are started from the suspension bracket of the mold - they are inserted into the hole on the front side of the mold and are also fastened with clay or ceresin at the source, only then at the end of each section. To ensure the selected contact with the electrically conductive layer, the conductor must be closely adhered to the mold: it is pressed against the plane by the blade of the knife. After the conductor has been laid, its other end is again inserted into the suspension hole of the mold, only then the suspension is fixed - an insulated conductor whose end is cleared of insulation along a length sufficient to contact the ends of the conductor laid in the form. Then the suspension wire in the form of a hook is bent.
Suspension of flat forms is best served by a 1-core copper wire with chlorovinyl insulation. Volumetric forms - a soft multicore wire with rubber or other (reliable) insulation, protecting the wire from the electrolyte. The cargo can be pieces of porcelain, glass, glazed also non-porous ceramics. And that the latter did not get metal (this is possible, if they get graphite dust), they cover with varnish or wax. The cargo must not be electrically conductive. In this regard, they are suspended on the molds after the application of the electroconductive layer.
Loading forms into the bath is done at an angle to the surface of the electrolyte, thereby facilitating the removal of the atmosphere from the narrow places of the mold. Then the flat form placed in the electrolyte is placed horizontally, so that the remaining air bubbles are removed with a soft brush. Reduce the trapping of bubbles, fill the mold with alcohol before loading. The forms are always pre-arranged in a location that allows the atmosphere to go up. The closed volumetric forms are filled with electrolyte little by little, evenly displacing the atmosphere from them. Deeply profiled rooms are kept so that the electrolyte, slowly pouring into them, would displace the atmosphere.
The initial current density is set to a minimum, while it does not cause any burn-up of the conductors that are connected to the conductive layer. This is maintained until the metal is completely tightened. Only then go to the working density - it is already safe.
Metalization of lace is a particularly common occupation in home electroforming. In a metallized state, finely ornamented laces suggest filigree, moreover, highly artistic. They can brighten up a variety of art products. Tulle laces, thin in pattern, most beautiful in combination with a translucent background, are also used in decoration as decorative lining. Guipure lace with a larger compared to the tulle net azhura are good for the direct production of various art products ( Figure 2, only also b ).
There is an electroforming in the preliminary finishing of lace, the build-up of metal, the subsequent electroplating of lace already on the product. Lace is first stretched on the frame, impregnated with paraffin, ironed between sheets of paper (excess impregnation is removed). Apply an electrically conductive layer (fine graphite), the excess of which is carefully blown off. After laying the conductors on the edge of the lace, they are fastened on a plastic frame made of thick wire with chlorovinyl insulation and also immersed with it in the electrolyte ( Fig. 2, c ).
The copper lined lace is treated with a brass brush. Of the metallized laces, the desired blank is cut out and also mounted on the article. Either the product itself is manufactured by imparting a corresponding shape to the lace preform. Solder them in the usual way - with the use of tin-lead solder. Electroplating consists in applying on the lace decorating layer of silver, gold or oxidizing them in the appropriate tone.
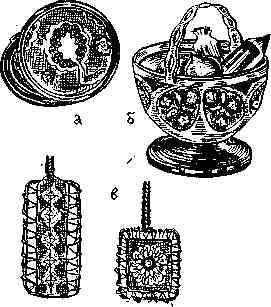
Fig. 2. Metallization of laces: only - a powdered box, ornamented with a metallic tulle lace in the form of a lining on the lid; B - candy, the walls of which are made of metallized lace; Only - pulling the lace conductor
In a similar way, with only small differences, production of, for example, herbariums, press molds for pressing acrylate products, metal coating of plants as fruits, and wooden things as feathers of birds is made. We will dwell in detail only on the latter.
Covering with metal products from wood, feathers of birds, having decorative meaning in the interior of the apartment. Such items will look cast metal. Products from logs pre-cooked in wax or paraffin, ceresin, ozocerite or other wax mixtures - eliminate hygroscopicity (it absorbs the electrolyte). Graph. Conductors are installed on them.
Suspend the load. The mold is loaded into the bath. In the same way, the feathers of birds are covered with metal, only not steaming them in wax or paraffin, just immersing them in a molten composition. The same graph, attach the conductor is also a load, lowered into the bath.




Comments
Commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.