| Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
METHOD OF GALVANIC COATING
Ilya Loskutov
![]()
Work with copper electrolytes has its own specifics. Let's start with the basic position of the direct application of copper coatings: in order to avoid the contact release of copper on the surface of the workpieces, they can be loaded into the electrolyte only under current, otherwise a strong adhesion of the sludge to the base material is not ensured. The initial current density also plays an important role in this situation. This is due to the fact that, with an excessively high current strength, even dense but crudely crystalline precipitates are formed, which in the future will lead, in the chosen case, to obtaining undulating or tubercular coverings of unpredictable thickness. At extremely low current density, the rapidity of the formation of the electroplating coating will lag behind the rate of release of contact-separated copper, which subsequently causes flaking of the coating.
For each variety of copper plating electrolytes, there is a specific, strictly defined, optimum current density. In practice, the optimal current density is selected according to the appearance of the coating received and the speed of its education. If there is a relatively small skill, which is acquired very quickly, a similar means of controlling the course of the process ensures the production of coatings of very high quality. When the galvanic process is correctly selected, the deposited copper layer has a solid color, a uniform fine-crystalline structure. With excessively impressive current densities, the copper layer is obtained with coarse grains of metal, also a characteristic brick-red color. About the final defect it is customary to express that the "sunburn" of the coating results. Exceeding the current intensity, in addition to the appearance of the decay, can lead to passivation of the anodes.
At the same time, a snow-white, greenish-blue or brown, smearing, easily erasable plaque is observed on the surface of the latter, which prevents the normal process of dissolution of the metal. At the same time, copper salts contained in the electrolyte are consumed to raise the coating, which leads to an instability in its chemical composition.
So, for the source, you need to prepare an electrolyte. On 1 liter of electrolyte it is necessary:
Copper vitriol - 60 g ;
Sugar refined sugar - 90 g ;
Caustic soda - 45 g ;
Alcohol - 5-10 ml .
The electrolyte is prepared in a strict sequence: copper sulphate is dissolved in 200-300 ml of water, sugar is added to it. Separately, in 250 ml of water, sodium hydroxide dissolves. Further in a solution of caustic soda in small portions with constant stirring, a solution of copper sulfate with sugar is added. Water is then added until a 1 liter solution is obtained. For the chosen dissolution of components, it is better to warm the water up to a temperature of 35-40 degrees. As a galvanic bath, you can use an ordinary jar. After preparation of the main electrolyte, 5-10 ml of alcohol is added to it. The presence of alcohol significantly improves the quality of the deposited copper, making the coating structure more dense also significantly reduces the grain of the metal. The finished electrolyte has a dark, saturated blue-green paint (such a paint can only be seen with a very small amount of electrolyte on the bottom also on light, but it seems that it is approximately black).
These proportions are taken from the chapter "Chemical also electrochemical methods of processing details" of the book "ABC of sudomodelism" (Dregalin AN Poligon S.-P. 2003) . But I think that you can experiment with the composition of the electrolyte.
To prepare the electrolyte it is strongly recommended to use not ordinary water from the tap, but distillate. It is also better to use no technical copper sulfate (fertilizer), but chemically neat crystalline hydrate of copper (the same copper sulfate) qualification is not lower than 4 . This is sold in stores of chemical reagents. Corrosive soda can be found in the same place.
Electrolyte maintenance is also a very significant issue. With the passage of the pores, a slurry is formed in the electrolyte composition, which significantly worsens its characteristics. Therefore, you should periodically pass the solution through a filter of 2 layers of tissue with a thin napkin between them. The same is recommended to be done immediately after the preparation of the electrolyte. In the interruptions of work, the bath (the jar) should be closed with a leakproof lid to prevent dust, debris, and evaporation of water from entering (and, as if, consequence, a violation of the concentration of components).
Next, we collect the electrical circuit. The electrode connected to the "+" (anode) acts from the copper sheet, for uniform coloring of the part twisted into the cylinder (see figures). The electrode connected to the "-" (cathode) is connected to the painted part. To remove the parameters in the electrical circuit, you can also turn off the ammeter and voltmeter. Schematic diagram is shown in the figure.
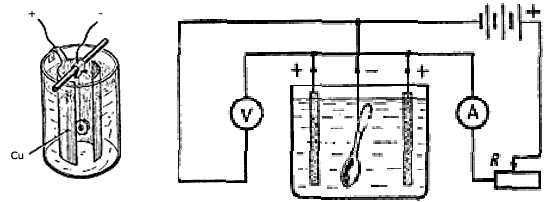
Circuit connection diagram
|
Cathode - painted part |
Anode |

How it looks in practice
To cover the part with a layer of copper, it is necessary to provide the required current density of about 0.5 (1-2) A / dm 2 (in different sources in different ways). Again, the current density depends on several factors, such as the size of the part, the composition is also the temperature of the electrolyte, the purity of the reaction (the purity of the water is also organic reagents), the reaction time. Therefore, most likely, each will have to choose the current itself with the help of variable resistance. To do this, in the circuit between the current source and the anode, it is necessary to provide an alternating resistance (tuning resistor or rheostat, which is preferable). As a source of current, you can use any source of constant current with an output voltage of up to 10 volts . In this sample we used the old charger for the Philips mobile phone with 4.2 V 770 mA output characteristics.
The current density is calculated by the formula: i = I / S
In what room: I - current power; S is the total surface area of the part to be painted.
With the strength of the current is more and less clear - it is set by the output parameters of the current source also by the variable resistance.
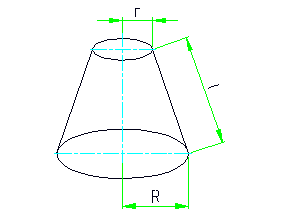
Let's find the total surface area of the painted part. Consider a previously made copy of the 24- pound cannon-carronade for the corvette "Olivuţa" .
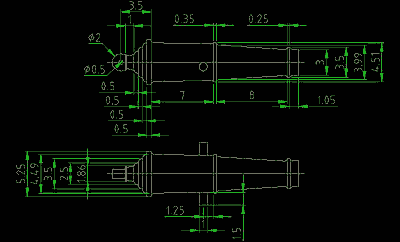
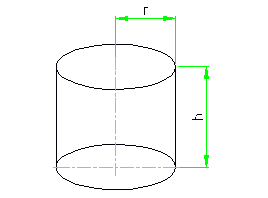
In this case, the complex figure of the gun consists of several simple figures - truncated cones also cylinders. But it is also difficult to calculate exactly the area of its surface - you can simply imagine it in the form of a cylinder with an average diameter and also calculate the approximate surface area, which for our work will be sufficient.
But you can also make more accurate calculations by dividing the part into geometric primitives and also calculating the area of the outer surface of each.
The total surface area of the cone is given by the formula: S = 3.14 * (R 2 + r 2 + l * (R + r)) ;
The area of the lateral surface of the cone: S = 3.14 * l * (R + r) ;
The total surface area of the cylinder: S = 2 * 3.14 * r * (r + h) ;
The area of the lateral surface of the cylinder: S = 2 * 3.14 * r * h ;
|
|
Substituting dimensions in the formula, we get the area of our part 0,03 dm 2 .
Hence the required current power: I = i * S = 0.5 (required current density) * 0.03 (part area) = 15 mA
And from here we get the required circuit resistance: R = U / I = 4.2V / 15mA = 280 Ohm
But the internal resistance of the electrolyte is not taken into account here, so in practice, the external resistance should be less than the calculated one. In my case, the optimal characteristics of the circuit were as follows: the above mentioned current source, counteraction in the anode circuit equal to 220 Ohm . It's time to paint the part for 17 minutes . As a galvanic bath used a glass jar from under the feeding of 150 ml , the area of the copper plate (anode) = 49 cm 2 , the temperature of the electrolyte room ( 18-22 0 C ).
If you do not have an ammeter or there is an alternating resistance that can not be determined with an accuracy of at least 10 ohms , then you can determine the required current on the eye as follows: for an extremely strong current, hydrogen is released at the cathode in the form of clearly visible bubbles (the so-called "boiling" "). These bubbles prevent the deposition of copper at the anode. In this case, the part is covered with a dark brown coating, easily rubbed fingers. Therefore, it is necessary to decrease the current (increase the resistance) until the hydrogen evolution becomes noticeable (hydrogen is released at any current strength), i.e. As if only bubbles did not become noticeable in any way, we can stop on this current strength also further change it (if necessary) by orienting ourselves in the appearance of the part.
After the part is taken out of the electrolyte, it must be thoroughly washed with running water. As a result, we get a painted part of matte copper color.
Next, to attach a detail of gloss we grind it thoroughly with a cloth with GOI paste or chalky chalk (tooth powder). We again rinse (we wash off the remnants of the chalk and also the GOI paste), and we also obtain the final result.
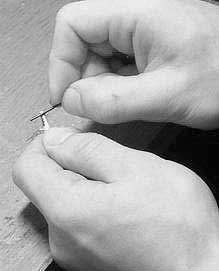
Some tips on the quality of the surface of the painted part. For a more even deposition of copper, the surface of the part must also be smooth and defatted. It is known that the part has a microporous surface during casting - these micropores must also be disposed of. If the material of the casting is soft (for example, tin), then this can be done with a steel needle, rolling or rolling it along the part, as shown in the figure.
At last, I think, it is necessary to describe the prospects of this technology. With the help of this copper-plating technology, it is possible to manufacture hollow parts from copper, for example a ship bell. For this, it is possible to make of any low-melting material the boll of the bell is also exactly similar to covering it with copper (but with a thick layer). The next step is scrupulous smelting of the material of the pig. But, undoubtedly, this requires a more complete layer of copper, and this time it will take considerably more than 17 minutes .
This technology can also be used to cover various carvings, gold, copper or bronze. In this method of galvanization, you can almost any color - you just need to experiment with the composition of the electrolyte and the current.
Creator: Ilya Loskutov





Comments
Commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.