| Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
Navigation: => |
Home / Patent catalog / Catalog section / Back / |
|
INVENTION
Patent of the Russian Federation RU2142066
![]()
METHOD OF ACCUMULATION OF ELECTRICITY
The name of the inventor: Kuznetsov Gennady Petrovich
The name of the patent holder: Kuznetsov Gennady Petrovich
Address for correspondence: 410041, Saratov, PO Box 723, Kuznetsov Gennady Petrovich
Date of commencement of the patent: 1997.06.25
The method is designed to accumulate excess electricity in power grids and can be used by electrolysis of a hydrogen-containing compound for the production and storage of hydrogen. The latter is intended to be used as fuel in power plants of standby generators, put into operation during peak hours in power systems. The electrolysis process is carried out by electrochemical anodic dissolution of a substance replacing hydrogen in a hydrogen-containing compound with the cathodic depletion of hydrogen cations and the precipitation of a chemical compound. The resulting compound is subjected to hydrolysis to obtain an initial amount of a hydrogen-containing compound and a solid product. The aqueous solution of the solid product is subjected to reversed polarity electrolysis to recover the initial amount of the material while additional hydrogen production is carried out. The method makes it possible to intensify and cheaper the process of obtaining hydrogen, while ensuring environmental safety.
DESCRIPTION OF THE INVENTION
The invention relates to the field of electric power engineering.
It is known that nuclear and thermal power plants with large units designed for high steam parameters should work as far as possible in a stable mode, since starting and stopping their units requires hours or even days. Electricity producers use a variety of methods to motivate consumers to more evenly receive electrical power. For example, in the UK in the winter time, for electricity consumed from 11 to 12 o'clock in the afternoon, a fee is almost five times higher than at night.
However, statistics show that in many power systems, the total electricity demand during certain periods of the day has significant differences - from a minimum during idle hours of energy-intensive equipment to a peak at peak hours, when the demand for electricity is about one-third greater than the production capacity. Periodic unloading and stopping of power plant equipment leads to increased wear and tear, loss of profitability and reliability and, as a result, to more frequent repairs. Overexpenditure of fuel while only, for example, in the Mosenergo system reaches one hundred thousand tons of equivalent fuel for the heating season.
Specialists in the field of electric power industry see only two ways to solve the problem of satisfying the demands of electricity consumers. The first method is based on increasing the capacity of power plants until the "peak" loads completely overlap. The second method involves the construction of special stations, which must produce the necessary amount of additional electricity during those peak hours. It is obvious that the first solution to the problem is not economical. To solve the problem of the second option, two types of power plants are used in the world practice: hydroaccumulating (PSPs) and gas turbine installations.
Both those "and others" are "spinning" in a matter of minutes and can produce a significant amount of additional energy. However, it is necessary to bear in mind a number of serious problems in the implementation of projects for the construction of maneuvering capacities. Despite the tempting advantage of pumped storage power plants capable of generating cheaper energy due to the discharge during peak hours through their turbines from the upper reservoir to the lower whole lake of water, which is again pumped to the upper reservoir by the pumps during the periods of minimal electricity consumption in the power systems, Special sites are required, which are often impossible to match in the conditions of a flat terrain, where most of the energy consumers are concentrated. Gas turbine stations in their work irrevocably destroy fuel, while polluting the atmosphere with harmful combustion products.
Specialists-heat engineers know such a wonderful type of fuel, which does not pollute the atmosphere of the Earth. It's hydrogen. The product of hydrogen combustion in power plants in which oxygen is used as an oxidizer is pure water vapor.
In the book J. Renewable Energy Sources (Moscow, Energoatomizdat, 1990), various ways of accumulating energy are described: chemical, thermal, electric, in the form of potential or kinetic energy. One of the types of chemical storage is the production of hydrogen by electrolysis of water using any current source. In the form of a gas, hydrogen can be stored, transferred to a distance and burned to produce thermal energy. The most proven way to produce pure hydrogen is electrolysis of water, but the efficiency of this process is about 60 percent. Part of the loss is associated with the occurrence of gas bubbles near the electrodes, which impede the movement of ions in the electrolyte and increase the overall resistance of the electrical circuit.
In order to intensify and reduce the cost of production of hydrogen on an industrial scale, a set of technological methods is proposed in specially designed devices for conducting electrochemical and purely chemical reactions, ensuring environmental safety and not requiring the destruction of non-renewable fuels.
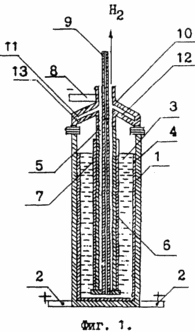
1 shows one embodiment of an apparatus for dehydrogenating a hydrogen-containing compound. The vessel 1, whose walls are in electrical contact with the bus bars 2 intended for this process for the anode current, is filled with a hydrogen-containing compound 3, for example ethyl alcohol. On the inner walls of vessel 1 there is an electroextract of substance 4 capable of replacing hydrogen in a hydrogen-containing compound, for example sodium. In the center of the vessel 1, a steel pipe 5 with apertures 6, which has been muffled from below, is located, which is intended in this process for removing hydrogen from the vessel 1. [ The pipe 5 is insulated from the hydrogen-containing compound 3 by a diaphragm 7 of a porous material having good electrical conductivity, sufficient density, mechanical strength and chemical resistance, for example from an asbestos fabric with a woven metal mesh. For the preparation of asbestos fabric is suitable white alkali-resistant long-fiber chrysotile asbestos. The pipe 5 has an electrical contact with the bus bars 8 designed for the cathodic current during dehydrogenation of the hydrogen-containing compound. In the center of the lower, muffled part of the pipe 5, a pipe 9 is isolated from it, intended to introduce into the vessel 1 a hydrogen-containing compound 3 to be dehydrogenated. The lid 10 electrically insulated from the vessel 1 has a cavity 11. The inner wall 12 of the lid 10 has valves 13 that separate the interior of the vessel 1 from the water supply pipes. The metal of the wall 12 of the lid 10 is a conductor of a constant electric current from the bus bars 8 to the metal walls of the pipe 5, thereby providing power to the cathode, the role of which is performed by the pipe 5 during the dehydrogenation of the hydrogen-containing compound 3. The described version of the device for dehydrogenating a hydrogen-containing compound is also suitable for two additional processes : For the hydrolysis of the product formed during dehydrogenation of the hydrogen-containing compound, as well as for the electrolysis of the aqueous solution of the solid hydrolysis product.
 |
 |
 |
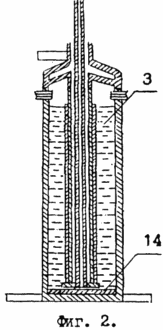
In Fig. 2, vessel 1 is depicted at the time of completion of the hydrolysis of the product formed during dehydrogenation of the hydrogen-containing compound. At the bottom of vessel 1, sodium hydroxide 14 precipitated, and ethanol was placed on top of it.
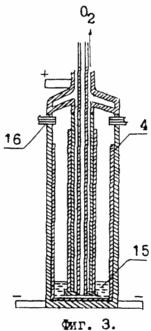
In Fig. 3, vessel 1 is depicted in the final stage of the electrolysis of aqueous solution 15 of sodium hydroxide 14. The bus bars 2 in this process are conductors of the cathode current, and the bus bars 8 are intended for supplying the anode current. The pipe 5 in this process is designed to collect oxygen, and hydrogen is withdrawn from the interior of the vessel 1 through the reduction gears 16.
THE COMPLEX FOR HYDROGEN PRODUCTION WORKS AS FOLLOWS:
An ethanol is introduced into the vessel 1 on which walls an electrocrack of sodium is placed 4. At the boundary between the ethyl alcohol 3 and the electroextract of sodium 4 (see Figure 1), a chemical substitution reaction in each molecule of an alcohol of one hydrogen atom of an alcoholic hydroxyl group is started by one atom Sodium, which results in the formation of sodium ethoxide and releases hydrogen gas. Ethylate of sodium dissolves in ethyl alcohol, as a result of which the solution acquires an electrical conductivity conducive to the beginning of anodic dissolution of the electroextract of sodium 4. Sodium separated from the layer of electroextract 4, interacts with ethyl alcohol throughout its mass, as a result of which the rate of the chemical substitution reaction of hydrogen atoms in the alcohol hydroxyl group Molecules of ethyl alcohol by sodium atoms increases in comparison with the heterogeneous character of the reaction at the initial stage. The hydrogen cations are discharged on the metal of the tube 5, which is a cathode in the process, and molecular hydrogen enters through the holes 6 inside the pipe 5 and is then removed from the vessel 1 for use by the consumers. After completion of the substitution reaction of the hydrogen atom in the last molecule of ethyl alcohol in vessel 1, a solid product is found - sodium ethoxide. Dehydrogenation of 92 parts by weight of ethyl alcohol with 46 parts by weight of sodium gives 136 parts by weight of sodium ethoxide and 2 parts by weight of hydrogen.
To restore the initial amount of ethyl alcohol in vessel 1, it is necessary to carry out the next stage of the technological process - hydrolysis of sodium ethoxide. The amount of water required for hydrolysis is introduced into the vessel 1 through the valves 13 of the lid 10. As a result of the interaction of 136 parts by weight of sodium ethoxide with 36 parts by weight of water, 92 parts by weight of ethyl alcohol and 80 parts by weight of sodium hydroxide are formed (see FIG. After the removal of ethyl alcohol from vessel 1, a solid product remains in it - sodium hydroxide 14.
To separate sodium from sodium hydroxide, the next step of the process is the electrolysis of an aqueous solution of 15 sodium hydroxide (see Figure 3). To form a twenty-five percent sodium hydroxide solution having the maximum electrical conductivity, 240 parts by weight of water are added to vessel 1 through the cavity 11 of the cover 10 to 80 parts by weight of sodium hydroxide. Dissolution of sodium hydroxide in water is carried out with the release of a large amount of heat, which leads to a sharp increase in the temperature of the solution 15. To place the electroextract of sodium on the walls of the vessel 1, electrolysis of the sodium hydroxide solution must be carried out by connecting the anode current through the bus 8, and the cathode current through the bus 2. On the cathode, whose role in the process of electrolysis is performed by the metal of the walls of the vessel 1, hydrogen cations are discharged. Molecular hydrogen creates a protective atmosphere for the electroextract of sodium, and the excess hydrogen is removed from the vessel 1 through the reducers 16. The oxygen anions are discharged on the metal of the tube 5, and molecular oxygen through the holes 6 enters the tube 5 and then is discharged through this tube from the vessel 1 to the consumers . After completion of the electrolysis of 80 parts by weight of sodium hydroxide dissolved in 240 parts by weight of water, 46 parts by weight of electroextract of sodium are deposited on the inner walls of vessel 1, 246 parts by weight of oxygen are discharged through pipe 5, and 28 parts by weight of hydrogen are discharged through reducers 16. To start a new cycle in vessel 1, it is necessary to introduce, through pipe 5, the initial amount of ethyl alcohol restored by hydrolysis of sodium ethoxide in another vessel.
Thus, after the completion of the three process steps described above, the initial amount of ethyl alcohol and sodium was restored. The only consumable is water. The weight of the hydrogen produced is 1/9 of the weight of the consumed water, and the remaining 8/9 parts of the weight of the consumed water is oxygen. In order to obtain the necessary amount of hydrogen and oxygen per unit of time, the capacity of the vessels connected to the battery is multiplied by the number of batteries.
THE SOURCE OF INFORMATION
1. J. Twydell A. Weir. "Renewable energy sources", Moscow, "Energoatomizdat", 1990, pp. 360-361, 364-365.
CLAIM
A method for accumulating electricity in power networks by electrolyzing a hydrogen-containing compound for the production and accumulation of hydrogen intended to be used as fuel in power plants of standby generators put into operation during peak hours in power systems, characterized in that the electrolysis process is conducted by electrochemical anodic dissolution of the substance Replacing hydrogen in a hydrogen-containing compound with the cathodic depletion of hydrogen cations and the precipitation of a chemical compound that is hydrolyzed to produce an initial amount of a hydrogen-containing compound and a solid product whose aqueous solution is subjected to reverse polarity electrolysis to recover the initial amount of the substance used in the replacement of hydrogen In a hydrogen-containing compound, while simultaneously obtaining additional hydrogen.
print version
Date of publication 24.03.2007gg




Comments
When commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.