| section Home
Production, Amateur Radio amateur Model aircraft, rocket- Useful, entertaining |
Stealth master
Electronics Physics Technologies invention |
space Mystery
Earth Mysteries Secrets of the Ocean Stealth section Map |
|
| Use of material is permitted for reference (for websites - hyperlinks) | |||
Navigation: => |
Home / Products Patents / In the section of the catalog / back / |
|
INVENTION
Russian Federation Patent RU2147873
![]()
Suppositories and method for the prevention and treatment of diseases, SEXUALLY TRANSMITTED
Name of the inventor: Seda Grigoryan Surenovna; Ershov Felix I.
The name of the patentee: Seda Grigoryan Surenovna; Ershov Felix I.
Address for correspondence: 125167, Moscow, ul.Stepana Suprun, 3/5, kv.72, SS Grigoryan
Starting date of the patent: 1999.06.11
The invention relates to medicine, namely to virology, immunology, pharmacology and gynecology. Suppositories and proposed a method of preventing and treating diseases, sexually transmitted diseases. Suppositories contain the interferon or interferon inducer or a combination thereof in a ratio of 2: 1 - 3: 1. Prophylactic and therapeutic use of suppositories containing IIFN and IFN - effective means of non-specific antiviral action, preventing viral replication and promotes their elimination.
DESCRIPTION OF THE INVENTION
The invention relates to medicine, namely to virology, immunology, gynecology and pharmacology regarding the development of the method and of creating an agent for preventing and treating diseases, sexually transmitted diseases, which can be used to prevent virus infection or other intracellular agents during sexual intercourse (simplex viruses 1 and herpes simplex type 2 (HSV-1, HSV-2), cytomegalovirus (CVM), Epstein-Barr virus, papillomavirus, hlamidini, mycoplasma, and various combinations thereof), and a treatment of diseases caused by them. It is possible to prevent using the proposed method and means of sexual transmission of hepatitis B, C and HIV infection. Furthermore, the invention may be used for the prevention of fetal infection in women before planned pregnancy.
For the treatment of the above infections along with traditional chemotherapy drugs and antibiotics are widely used systemic administration of different interferon inducers (IIFN) having the best combination of non-specific antiviral action, immunomodulatory and antiproliferative activity.
An object of this invention is rapid and effective increase of nonspecific resistance mucosa and underlying tissues of the genital tract of women to infection by different viruses and other intracellular agents or improvement in an already infected site and the presence of disease.
To solve this problem the local production stimulation conducted interferon (IFN) in the vaginal tissue and the cervix, which are used for pessaries comprising IIFN IFN preparations. The local prophylactic use of vaginal suppositories IIFN interaction with the surrounding healthy tissue causes the synthesis of a variety of ![]() IFNov and other inflammatory cytokines in the gate potential infection. By non-specific, i.e. directed against all taxonomic classes of antiviral action of a mixture of synthetic viruses
IFNov and other inflammatory cytokines in the gate potential infection. By non-specific, i.e. directed against all taxonomic classes of antiviral action of a mixture of synthetic viruses ![]() endogenous hozyainospetsificheskih IFNov, IIFNov increase non-specific resistance of the treated tissue to the effects of different viruses and their associations, preventing their reproduction at the gates of infection, further penetration and spread in the body. Of particular importance in this context acquires induced endogenous
endogenous hozyainospetsificheskih IFNov, IIFNov increase non-specific resistance of the treated tissue to the effects of different viruses and their associations, preventing their reproduction at the gates of infection, further penetration and spread in the body. Of particular importance in this context acquires induced endogenous ![]() Which, as known, has a more pronounced local antiviral effect.
Which, as known, has a more pronounced local antiviral effect.
Thus, local IIFN prophylactic use in combination with interferon as pessaries gate creates a potential infection kind nonspecific interferon barrier replication of various viruses. Moreover, the prophylactic use of vaginal suppositories can be the right time to prevent infection of both sexual partners.
The use of vaginal suppositories containing IIFN, with the purpose of treatment potentiates the effect of regular use IIFN, IFN and other chemotherapy drugs, can increase the concentration of various types of interferon in the lesion, creating the possibility of a direct impact on the body - the target.
Simplicity and ease of use of such universal suppositories with prophylactic or therapeutic purposes helps to reduce the spread of viral sexually transmitted diseases and increases the effectiveness of their treatment.
The prototype of the invention may be systemic (in / m, / in, oral) application IIFN in the treatment of genital herpes, a digital computer infection, chlamydia, mycoplasmosis, candidiasis, which in recent years in the vast majority (75%) presented different combinations of mixed infection and accompanied by marked inhibition of IFN system and immunity. By non-specific antiviral and immunocorrecting IIFN action in these diseases and improve clinical efficacy aetiological recovery to an average of 92%, and corrects the normalized parameters and immune interferon (1 - 8).
The present invention differs from the prior art in that it is a vaginal suppository, IIFN content in combination with IFN and intended for the local induction of the natural (endogenous) infection in IFNov gate not only therapeutic, but also and above all, as a prophylactic measure. Moreover, the use of vaginal suppositories does not exclude the systemic exposure of the mixture induced IFNov and cytokines, which creates an additional advantage of their antiviral and immunomodulatory effects on the body as a whole.
As a means for the prevention and treatment of viral and other intracellular infections, sexually transmitted infections, vaginal suppositories are available, consisting of IIFN-Poludan and interferon (IFN, realdiron) in a ratio of 2: 1 - 3: 1.
Suppositories weighing 1.0 g are prepared based on cocoa butter or hard pork fat, 1.3 - 1.5 weight active ingredients which make up.
Poludan - poliribonukleotidny complex Polyadenylic and polyuridylic acid (Poly AU) (Pokrovsky plant biologics Biosaf). The high IIFN, the body induces the synthesis of ![]() IFN types.
IFN types.
It is used for the treatment of viral diseases of the eye, herpetic and adenoviral conjunctivitis, keratokonyuktivitov, keratitis and keratoiridocyklites in the form of eye drops, subekonyuktivalnyh and retrobulbar injections. It has a broad spectrum antiviral effect and has no toxic effect on the body.
Interferon - (IFN, realdiron) human recombinant IFN - (NGO "Vector" Novosibirsk) lyophilized powder in vials of 1,000,000 - 3,000,000 IU by intramuscular injection at various viral diseases and cancer.
Under the action of interferon in the composition of additional stimulation occurs suppository products IFNov poludanom (priming effect - superinduktsii), which increases the effectiveness of the antiviral effect of the drug.
Below is a summary of the test results of the antiviral action reaferon, Poludan and combinations thereof at various concentrations in cell culture human fibroblasts M19 embryo when infecting a reference to determine the antiviral action of IFN vesicular stomatitis virus (VSV immediately after drug administration (Table 1) and after 2 chasa after treatment (table 2).
The data in Table 1 shows that when infecting Force immediately after treatment of culture the greatest effect antiviral defense combination reaferona and Poludan concentrations 10000 - 5000 IU / ml and Poludan concentrations 10000 - 5000 IU / ml and 20 - 10 pg / ml, respectively, constituting 60% after 48 hours falls to 10% and further not determined.
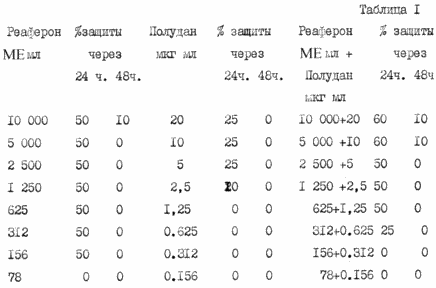
Upon infection by Air culture 2 hours after treatment (. Table 2) is determined 100% protection effect reaferona combination (10,000 IU / ml, 1,250 IU / ml) and Poludan (20 - 2.5 mg / mL) that is kept at the maximum level in for 48 hours and 7 days is determined to follow.

These data indicate that both Poludanum and reaferon exhibit pronounced protective effect (75% - 50%), but the best quick and effective antiviral effect (100%) provides the combination of Reaferon and Poludan proposed in the vehicle, which is experimental basis whether it should use in the prevention and treatment of viral and other intracellular infections, sexually transmitted diseases.
Similar vaginal suppositories may be prepared using other approved for clinical use IIFN: amiksina, tsikloferona, Neovir, ridostine, larifana, but also a number of formal preparations - dibasol, papaverine, caffeine, dipyridamole, who demonstrated the presence of IFN-inducing and antiviral activity.
The effectiveness of the proposed vaginal suppositories were tested in the complex treatment of chronic inflammatory diseases of the mixed virus, mycoplasma chlamydial etiology in human volunteers (n = 15).
Suppositories used for suppositories scheme-2 at night every day for 10 days. In the course of 20 suppositories.
Clinical efficacy was expressed in the reduction of terms disappearance of clinical symptoms (for 3 - 4 days, in the control - 7 - Day 9), normalization colposcopic picture (for 5 - 6 days in 80% (as in the control to 20%), reducing the frequency allocation HSV-1 and HSV-2 (5.6-fold; in control - 2.4 times), DCM (2.4 times, unchanged in the control) chlamydia disappearance (at 92.9%, 55.6 % in the control) and microplasmas (all while maintaining in the control).
The use of vaginal suppositories and increased IFN - inducing ability of white blood cells of patients
(Table 3).

Prophylactic use of vaginal suppositories (1 per night 24 - 48 hours, in the course of 10 - 20 suppositories) in women volunteers before planned pregnancy decreased antibody titers to viruses HSV-1, HSV-2 and TSMB, contribute to the successful conception and pregnancy and birth, preventing the possibility of reactivation of viral infection and intrauterine infection.
The data show that the prophylactic and therapeutic use of suppositories containing IIFN - IFN - effective means of non-specific antiviral action, preventing viral replication and promotes their elimination.
USED BOOKS
1. Kozlov VI, Puchner AF Viral, chlamydial and genital mycoplasma disease. Medicine 1995, p. 314.
2. Ershov FI, LV Antonova, SS Grigoryan, Dry GT et al. Abnormalities in the interferon system in patients with virusassotsirovannymi and chlamydial infections. Questions of Virology, 1996, 4, 172 - 174.
3. Dry GT et al. Use of interferon inducers in the treatment of urogenital infections. Proceedings of the I Vserosijsky scientific-practical conference. Sochi. April 1996, 17 - 21.
4. Antonova LV, FI Ershov, SS Grigoryan et al. Interferon status of women with gynecological diseases complicated by viral, chlamydial and fungal infection. Bulletin of the Russian Association of Obstetricians and Gynecologists in 1996, 2, 80 - 83.
5. Antonova LV, FI Ershov, SS Grigoryan et al. A new generation of Immunopreparat for the treatment of associated virus diseases of genitals of women. III Russian National Congress "Man and Medicine" Moscow 1996, 67.
6. Tazulachova EB, Parshina OV, Guseva TS, Matveeva NS, Sukhih GT, Ershov FI, Effectiventss of Herpes virus, CMV and Chlamidia infections treatment with IFN inducers larifan and ridostion. Eurip.Cytokine Network 1996, v. 7, p. 651.
7. Grigorian SS Ospelnikova TP, Ershov FI The rational use edogenous IFN-system as antiviral agent. V1 Bienal Confer. on Antiinfective agents and chemotherapy 1996, Germany.
8. Ershov FI, Grigorian SS, Aufonova LV Interferon and JFN inducers Therapy of sexually transmitted diseases of women. European Cytokine Network 1996, v. 7, N 3, 651
CLAIM
1. Suppositories for treatment and prophylaxis of viral and other intracellular infections, sexually transmitted diseases, the base comprising an interferon inducer (IIFN) or interferon, or a combination thereof in a ratio of 2: 1 - 3: 1.
2. Suppositories according to claim 1, characterized in that they contain as the basis cocoa butter or lard solid.
3. Suppositories according to claim 1, characterized in that they contain as IIFN Poludanum at 100 - 200 micrograms.
4. Suppositories according to claim 1, characterized in that they contain as an interferon concentration reaferon 50000 - 200000 IU.
5. The suppositories according to claim 1, characterized in that they contain IIFN - Poludanum a concentration of 100 - 200 mg and at a concentration reaferon 50000 - 200000 IU.
6. Suppositories according to claim 1, characterized in that they contain as IIFN agent selected from the group amiksin, tsikloferon, neovir, ridostin, larifan or several of the preparations: Dibazolum, papaverine, caffeine, dipiridanol.
7. A method for the prevention and treatment of viral and other intracellular infections, sexually transmitted diseases, comprising administering IIFN, characterized in that the means in the form of suppositories, further comprising interferon, thus as IIFN Poludanum used at a concentration of 100 - 200 mcg interferon and reaferon at a concentration of 50,000 - 200,000 IU in a ratio of 2: 1 - 3: 1.
8. A method according to claim 7, characterized in that for the treatment of viral and other intracellular infections suppositories are administered according to the scheme 2 overnight suppository daily for 10 days.
9. A method according to claim 7, characterized in that for the prevention of viral infections and other intracellular suppositories are administered according to the scheme 1 suppository overnight rate at 10 -. 20 suppositories.
10. The method according to claim 7, characterized in that to prevent the possibility of viral infection or other intracellular infections during sexual intercourse 1 - 2 suppositories are administered within 30 - 120 min before or immediately after sexual intercourse.
print version
Publication date 09.01.2007gg




Comments
Commenting, keep in mind that the content and the tone of your messages can hurt the feelings of real people, show respect and tolerance to his interlocutors, even if you do not share their opinion, your behavior in terms of freedom of speech and anonymity offered by the Internet, is changing not only virtual, but real world. All comments are hidden from the index, spam control.