| Start of section
Production, amateur Radio amateurs Aircraft model, rocket-model Useful, entertaining |
Stealth Master
Electronics Physics Technologies Inventions |
Secrets of the cosmos
Secrets of the Earth Secrets of the Ocean Tricks Map of section |
|
| Use of the site materials is allowed subject to the link (for websites - hyperlinks) | |||
Navigation: => |
Home / Patent catalog / Catalog section / Back / |
|
INVENTION
Patent of the Russian Federation RU2120284
![]()
COMBINED ANTI-INFLAMMATORY, ANALGESIZING,
FEBRIFUGE
The name of the inventor: Uderer Mikhail Semenovich; Sofronov, Henry Alexandrovich; Pienyshkin Vladimir Ivanovich; Frizen Ivan Dietrichovich; Goryainov Valery Nikolayevich; Kovalenko Anatoly Alexandrovich
The name of the patent holder: Sofronov, Henry A.
Address for correspondence:
Date of commencement of the patent: 1997.07.25
The invention relates to medicine and relates to a combined medicament. The essence of the invention lies in the fact that it is used for pain syndrome of moderate intensity, and in the case of febrile conditions for colds and the group. The drug contains in certain weight percentages: acetylsalicylic acid, paracetamol, caffeine, ascorbic acid, starch, cocoa, citric acid, calcium stearate, talc, aeroisl, polyvinylpyrrolidone. The proposed means provides for the production of quality tablets, leads to an increase in the therapeutic effect and a reduction in the adverse effects of paracetamol and acetylsalicylic acid.
DESCRIPTION OF THE INVENTION
(EN) The invention relates to medicine, in particular to the manufacture of combined medicines used for pain of moderate intensity, and in cases of febrile conditions for catarrhal diseases and influenza.
Combined analgesic, antipyretic "Citramon P", containing acetylsalicylic acid, paracetamol, caffeine, cocoa, starch, citric acid, talc and calcium stearate is known [1].
One tablet of this drug has the following content of ingredients, by weight:
Acetylsalicylic acid - 41.4 - 45.8
Paracetamol - 31.1 - 33.3
Caffeine - 4.9 - 6.0
Cocoa 1.4 - 4.1
Starch - 10.0 - 15.0
Citric acid - 0,4 - 1,8
Talc - 1.0 - 3.0
Calcium stearate 0.5 - 1.0
The composition of the drug "Citramon P" includes paracetamol and acetylsalicylic acid, which can lead to side effects. Thus, paracetamol can be used for long-term use, especially in high doses, for nephrotoxic and hepatoxic effects [2]. It is known about the potential danger of using paracetamol in alcoholism and chronic liver disease [3].
It is also known that acetylsalicylic acid somewhat suppresses immunity, but also promotes the release of ascorbic acid from the body with urine [4]. When taking acetylsalicylic acid, nephrotoxic and hepatoxic effects are possible [5].
Both these preparations, being xenobiotics, reduce the antioxidant defense of the body. To reduce the possible adverse effects of paracetamol and acetylsalicylic acid, it is advisable to strengthen the antioxidant defense of the body by including a drug with antioxidant properties in the preparation Citramon P.
It is known that ascorbic acid (vitamin C) belongs to the group of water-soluble antioxidants. Ascorbic acid is involved in the detoxification of xenobiotics, contributes to increasing the body's immune defenses against infectious diseases [6].
The need for ascorbic acid increases with fever and inflammatory processes, when taking many medications.
Thus, the inclusion of ascorbic acid in the combined preparation "Citramon P" will enhance the therapeutic effect in its use and reduce the adverse effects of paracetamol and acetylsalicylic acid.
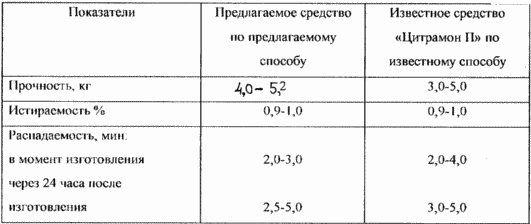
The proposed anti-inflammatory analgesic and antipyretic agent containing acetylsalicylic acid, paracetamol, caffeine, cocoa, starch, citric acid, calcium stearate and talc, further contains ascorbic acid, aerosil and polyvinylpyrrolidone with the following ratio of ingredients, by weight:
Acetylsalicylic acid - 38.0 - 42.0
Paracetamol - 28.5 - 31.5
Caffeine 4.5 - 5.5
Ascorbic acid - 7.5 - 9.2
Starch - 7.0 - 9.3
Cocoa 3.5 - 5.5
Citric acid - 0.6 - 1.0
Calcium stearate 0.4 - 1.0
Talc - 0.2 - 2.0
Aerosil - 0,2 - 2,0
Polyvinylpyrrolidone 0.2 - 2.0
New in the proposed composition of the drug, compared to the known, is that it additionally contains ascorbic acid, aerosil, polyvinylpyrrolidone and a new ratio of ingredients. The proposed ratio of active ingredients (acetylsalicylic acid, paracetamol, caffeine and ascorbic acid) and auxiliary substances (starch, cocoa, citric acid, calcium stearate, talc, aerosil and polyvinylpyrrolidone) is optimal and ensures the production of quality tablets and the proper therapeutic effect of the drug.
Aerosil and polyvinylpyrrolidone are allowed for use as auxiliary substances (ГФ IX, т. 2, с. 155). Their introduction provides a granulate with the desired properties and improves the process of tableting a new drug.
Failure to meet the limits of the ratio of any of the ingredients in the proposed drug, with respect to the declared limits of the ratios of all other ingredients, does not achieve a positive effect.
The limits of the ratio of the active substances determine the therapeutic effect of the proposed preparation. The limits of the proportions of auxiliary substances are proposed for the manufacturability of the proposed medicinal product, its stability during the shelf life and compliance with the requirements of the State Pharmacopoeia XI edition.
Reduction of starch (less than 7% by weight) leads to an increase in the disintegration of tablets (according to GF XI no more than 15 minutes), deterioration of acetylsalicylic acid dissolution (not less than 75% by weight in 60 min), and starch increase (more than 9.3% %) Leads to a decrease in the strength of the tablets for abrasion (by GF XI no less than 97%). Reduction of cocoa (less than 3.5% by weight) leads to a loss of marketable appearance, and an increase (more than 5.5%) leads to an increase in the disintegration time of tablets more than 15 minutes.
Reducing citric acid (less than 0.6% by weight) leads to a decrease in the strength of the tablets for abrasion, and an increase (more than 1.0% by weight) leads to an increase in the disintegration time of tablets more than 15 minutes.
The decrease in calcium stearate (less than 0.4% by weight) impairs the sliding properties of the tablet mixture, leads to mashing the press tool, and an increase (more than 1.0% by weight) is not allowed (according to GF XI, v. 2, p. 155 ).
The reduction of talc (less than 0.2% by weight) impairs the sliding properties of the tablet mixture, an increase in talc (more than 2% by weight) is undesirable (according to GF XI, the amount of talc in tablets should not exceed 3% by weight).
Reduction of aerosil (less than 0.2% by weight) and deteriorates the sliding properties of the tablet mixture, and an increase (more than 2.0% by weight) is undesirable (according to GF XI, the amount of aerosil in tablets should not exceed 10% by weight).
Reduction of polyvinylpyrrolidone (less than 0.2% by weight) does not allow to obtain granulates of a given quality, and an increase (more than 2.0% by weight) leads to an increase in the disintegration time of tablets more than 15 minutes.
Despite the increase in the amount of excipients (aerosil and polyvinylpyrrolidone) as compared with Citramon P, the total content of the proposed medicinal product (from 12.0 to 22.8 mass%) is lower than in Citramon P (from 13 , 3 to 24.9% by weight).
As follows from the above, only the declared intervals of the ratio of ingredients is optimal and allow to obtain a medicinal product with improved therapeutic properties as compared with "Citramon P" and meets the requirements of the normative documentation for medicines in force in the Russian Federation in all respects.
INFORMATION SOURCES
1. Pat. RF N 2034533, cl. A 61 K 31/62, 31/135, 31/615, Bul. N 13, 1995.
2. Instructions for the use of paracetamol. Approved by the Pharmacological Committee on 17.12.1992.
3. Polosky V.M. Aspirin, other salicides and paracetamol are non-prescription analgesics. - M .: Pharmateka, N 2, 1996.
4. Boltkajs Ya. Ya., Fateev V.A. Interaction of medicinal substances. - M.: Medicine, 1991.
5. Instructions for the use of acetylsalicylic acid. Approved by the Pharmacological Committee, 1983.
6. Melentieva TA, Taber AM Ascorbic acid and its derivatives are medicines. M .: Chemico-pharmaceutical industry, no. 2, 1991.
7. State Pharmacology of the USSR XI edition (GF XI). In two parts. - M .: Medicine, 1987 - 1989.
CLAIM
Combined anti-inflammatory, analgesic, antipyretic agent containing acetylsalicylic acid, paracetamol, caffeine, cocoa, starch, citric acid, talc and calcium stearate, characterized in that it additionally contains ascorbic acid, aerosil and polyvinylpyrrolidone in the following ratio of the ingredients, by weight:
Acetylsalcic acid - 38.0 - 42.0
Paracetamol - 28,5 -31,5
Caffeine 4.5 - 5.5
Ascorbic acid - 7,5 -9,2
Starch - 7.0 - 9.3
Cocoa - 3.5-5.5
Citric acid - 0.6 - 1.0
Calcium stearate 0.4 - 1.0
Talc - 0.2 -2.0
Aerosil - 0,2 - 2,0
Polyvinylpyrrolidone 0.2 - 2.0 t
print version
Date of publication 06.01.2007gg




Comments
When commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.