|
As you probably know fish do not fly and do not go - they swim. And naturally live in water. What kind of water is that - in which the fish live? What are its parameters and properties? What kind of water should be in the aquarium for your fish? About this and many other things we will discuss in this section.
We all know that water can be of different qualities and have different properties. Well of course except for distilled water. In different natural conditions, their water parameters. Any living organism throughout its life is inextricably linked with the environment, which is influenced not by its appearance, body structure, behavioral features. Fish, being the inhabitants of the aquatic environment, are affected by such factors as temperature, light, concentration of oxygen and carbon dioxide, osmotic pressure, acidity, stiffness and salt composition of water. Below we will get acquainted with all the above parameters in more detail.
Water itself is the living space of aquarium fish and plants and, depending on its properties, promotes the development of life processes in them or inhibits them. It contains various substances that give it such properties of interest to the aquarist as color, transparency, odor, as well as the stiffness values of DH and pH PH.
For the aquarium, a clean tap water containing tap water with DH = 5-20 degrees, KH = 2-15 degrees, PH = 6.5-7.5 is suitable for the life of plants.
Every aquarist should first of all be aware of, at least, the rigidity of water in his domestic water supply. And how do you know otherwise, does he pour the right water into the aquarium for his pets? Generally speaking, you can call the waterworks and get general information on this issue. But now in all the specialized stores are available for sale tests, with which you can easily make measurements of your water.
So, let's start to understand more in detail what parameters are at the water.
WATER ACID (HYDROGEN INDEX) PH
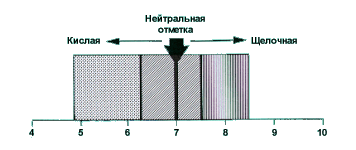
Any type of water we distinguish by the content of acids and bases. PH is the measure of the acid or base content. Acidity of water has a great influence on biochemical and biological processes and is important for fish. Ions in water are carriers of acidic or alkaline properties. If alkaline and acidic ions are contained in it in equal amounts, then the water reacts "neutrally", as it does (or at least should occur) with distilled water. Any water contains a certain amount of H2O molecules, separated into H + - cations (positively charged hydrogen ions) and OH - anions (negatively charged hydroxide ions). The total content of H + and OH ions in a liter of water has a constant value of 10-14 mol / liter (mole is the molecular weight in grams) measured at 25 degrees C. When the concentrations of both ions are equal, the content of each of them is 10 -7 mol /liter. So, neutral water has a concentration of hydrogen ions of 10 -7 grams per liter, that is, it contains 0.0000001 g of H-ions and exactly the same number of OH ions. Difficult and not obvious, is not it. This number is called the PH indicator (Pondus Hydrogenii). It is for clarity that they write completely not his, but only the logarithm of the hydrogen index without a negative sign, that is, simply "7". And accordingly, the hydrogen index of the neutral medium is abbreviated as "PH 7". In acidic water, this figure is lower than 7; In alkaline is higher than 7.
Depending on the acidity, water is classified as follows:
- PH = 1-3 strongly acidic water
- PH = 3-5 acidic water
- PH = 5-6 slightly acidic water
- PH = 6-7 very weakly acidic water
- PH = 7 neutral water
- PH = 7-8 very slightly alkaline water
- PH = 8-9 weakly alkaline water
- PH = 9-10 alkaline water
- PH = 10-14 strongly alkaline water
 |
| Most plants develop well in water, whose pH value is at the neutral point (7.0). At rates below 5.0 and above 8.5, they most often cease to grow or even die. |
For information, the acidity of tap water, as well as of water in running water, approaches PH = 7.
It should also be taken into account that the dissociation of water molecules, and, together with it, PH also depend on temperature. This fact is often overlooked even by experienced aquarists. Meanwhile, if measured at 18 degrees C PH = 7, then such water can not be considered neutral, since a neutral reaction at this temperature corresponds to a different value of PH.
The hydrogen index has an important general biological significance, and in the course of evolution in the majority of living organisms a number of mechanisms have been developed that ensure a relative constancy of this index in the cell. The role of this factor is determined primarily by its influence on the activity of enzymes and the state of other protein molecules. In addition, since most of the reactions in cells take place in an aqueous medium, the excess or deficiency of ions can significantly affect the course of various non-enzymatic reactions as well. This is the main reason that most cells belonging to a wide variety of organisms are able to live in a narrow PH range - from 6.0 to 8.0. However, even novice aquarists usually know that many fish quite painlessly suffer much greater deviations from the neutral reaction of water. This is explained by the fact that the body has a number of buffer systems that smooth the sharp fluctuations of the PH environment.
Buffer properties are not only the body fluids. A significant role in ensuring the relatively stable acidity of fresh waters is played by their carbonate system. Carbon dioxide not only dissolves in water, but, reacting with it, forms a weak acid HCO3. The presence of salts in the water strengthens its buffer properties, so that the same acidification of soft and hard water will cause in the first case a much more noticeable shift of PH. Even more powerful than the hard water, buffer properties, or, as they say, even more buffer capacity, has sea water.
The active reaction of water largely depends on the intensity of photosynthesis and the population of the reservoir by plant organisms. In photosynthetic processes occurring in the light, plants consume carbon dioxide, which causes an increase in PH. At night PH decreases, which is due not only to the lack of photosynthesis, but also to the release of CO3 during the respiration of plants. All this leads to very significant fluctuations in the active reaction of the medium in the reservoir during the day. These oscillations are especially great in a reservoir with a high content of plants. To reduce the sharp fluctuations in the acidity of water, it is recommended that the aeration of water with air is constant. This we will describe in detail in the section: Aeration and filtration.
Practical advice:
To lower the water PH, i.e. Make it more acidic, you can add to it peat, taken not elevations. You can also add a filtered extract obtained after boiling the peat. Be careful, because Excess of peat may be harmful, since it contains many tannic substances. Alkaline water can also be acidified by adding sodium biphosphate to it.
Practical advice:
To increase the PH of water, i.e. Make it more alkaline, you can add to it the usual drinking soda. Well, to alkaline acid water, you need to add a little sodium bicarbonate or mix acid water with alkaline tap water, i.e. To dilute. The latter method is most suitable and advantageous, because with the addition of fresh tap water, a lot of natural humic acids enter the water and the change in PH does not happen so drastically. Still it is worth remembering that for the content of aquarium fish is not suitable strong acid, acidic, alkaline and strongly alkaline water. The water must be either very weakly acidic, neutral, or very slightly alkaline. You can also use proprietary drugs to reduce PH - we are talking about a decrease because This is much more difficult to achieve than to increase PH. So be careful when using PH down, because The latter are often made on the basis of orthophosphoric acid. And as you know from practice, this acid keeps the pH at about 6.5, naturally depending on how much you used acids. Unfortunately, the use of orthophosphoric acid has a side effect - an increase in the phosphate level in the aquarium - to the great misfortune, yes, unfortunately for all aquarists. And as we know from practice, phosphates in water stimulate the growth of the simplest algae. Another way to reduce PH is to use hydrochloric acid. The exact amount of acid added to the water will always depend on the buffer capacity of the water. Simply, you add acid to the boundaries when the entire water buffer is exhausted. As soon as you achieve this, then it will be easy to reduce PH later. Just remember that water with a low PH has a smaller buffer capacity than water with increased PH. And you already know about the water buffer, read above. So draw conclusions. Be careful with the application of this method, the more it is associated with the use of acid - school chemicals probably remember, with acids you have to be careful.
Practical advice:
If you find that the PH of the water in your aquarium has changed dramatically in one direction or the other, do not add a large amount of soda or peat to the water immediately. Remember: A sharp change in the acidity of water can lead to the death of fish. Everything must be done gradually. And more on the same occasion. To avoid sudden changes in PH, change the water in the aquarium in small portions. Better a little and often, the less often and immediately more than half of the aquarium!
Now let's pass to an equally important parameter of water - its rigidity.
WATER RIGIDITY (DH)
Water hardness is one of the most important parameters of fresh water, on which the possibility of keeping and breeding fish and cultivating plants in the aquarium depends. It should be noted that its values for aquarium fish and plants can significantly differ from the values in natural reservoirs, because fish and plants have tremendous adaptability to environmental conditions, especially during the generation change process. Any fresh or salt water from natural water bodies contains more or less calcium ions. This is one of the most necessary elements: in aquatic crustaceans and mollusks it provides the hardness of shell or shell, in fish of the bone system. Calcium ions also play an important role in the regulation of osmotic pressure, which we will discuss below, and many other processes in the body. Thus, in some fish, the content of Ca in the blood depends on the degree of maturity of the sexual glands.
According to our standardization system, the hardness is expressed in mmol-equivalents of calcium ions (Ca ++) or magnesium (Mg ++) contained in 1 liter of water. 1 mmol-equ. Corresponds to a water content of 20.04 mg Ca ++ or 12.16 mg Mg ++.
In aquarium practice, the stiffness is denoted in degrees. One degree of rigidity (Russian or German) corresponds to 10 mg of calcium oxide (CaO) or 7.19 mg of magnesium oxide (MgO) in 1 liter of water and is equal to 0.35663 mmol-eq.
The stiffness is subdivided into temporary (carbonate) and permanent (non-carbonate). Carbonate hardness is due to the presence of calcium and magnesium hydrogen carbonates. When boiling, the hydrocarbonates are destroyed, and the ions Ca2 + and Mg2 + precipitate in the form of sparingly soluble carbonates. Over time, some of the precipitated carbonates dissolve again, especially in alkaline water with an index of PH = 8.3, which leads to an increase in the time stiffness. Stiffness, which persists after boiling water, is called a constant.
Basically, all tests to determine the hardness of water are made to measure the rigidity in degrees. We will not, and we will paint here in other units of measure, we will write down immediately in degrees.
So, depending on the rigidity of water is divided:
Very soft water
|
From 0 to 4 ° dN
|
Soft water
|
From 5 to 8 ° dN
|
Water of medium hardness
|
9 to 12 ° dN
|
Rather hard water
|
From 13 to 18 ° dN
|
Rigid water
|
From 19 to 30 ° dN
|
Very hard water
|
From more than 30 ° dN
|
In the literature on aquaristics, "German degrees of rigidity" is used most often when stating the rigidity. Below is a table of translations from German degrees to degrees in other countries and vice versa.
|
Him.
° dN
|
Engl.
° еН
|
Franz.
° fN
|
Americas.
° usN
|
CIS ° suN
|
| 1 German.
degree
|
1.00
|
1.25
|
1.78
|
17.8
|
7.15
|
| 1 Eng.
degree
|
0.798
|
1.00
|
1.43
|
14.3
|
5.70
|
| 1 fr.
degree
|
0.560
|
0.702
|
1.00
|
10.0
|
4.0
|
| 1 american.
degree*
|
0.056
|
0.070
|
0.10
|
1.0
|
0.40
|
| 1 rus.
degree
|
0.14
|
0,111
|
0.078
|
0,0078
|
1.00
|
Data in ppm (ppm per million), provided that the mass of 1 liter of water is 1 kg.
The rigidity of natural waters can vary within fairly wide limits and varies throughout the year. Increased rigidity due to the evaporation of water, decreases during the rainy season, as well as during the melting of snow and ice. The waters of the seas and oceans, as well as of reservoirs with soil consisting of calcium rocks, are the most stiff. Least hard water is found in water bodies that feed exclusively on atmospheric precipitation (provided that their soils do not contain calcium), in tundra and taiga waters, in forest reservoirs and in rivers flowing in areas with peat soils.
Practical advice:
Pay attention not the previous paragraph above - this natural method can be used in the aquarium.
To contain and breed aquarium fish, you need to maintain a certain water hardness. If the soil is coarse-grained sand and river pebbles, then the aquarium water will have more or less constant rigidity. We must also remember that in aquariums where fish and shellfish are kept, the stiffness gradually decreases, because calcium is used to build shells by shellfish, it is assimilated by plants and fishes.
What are the ways to reduce stiffness:
Practical advice:
1. First of all, distilled, rain or melt water can be added to the aquarium.
2. You can use such aquarium plants as elodea and hornwort.
3. By freezing. The water is poured into a low basin and placed in the frost or in the freezer. After the water half of the height of the vessel freezes, pierce the ice, water is poured, and the ice is melted.
4. By mixing with softer water. This too is clear.
5. By boiling water. The water is boiled for an hour in enameled dishes. Then cool and merge 2/3 of the top layer, in which the stiffness will be reduced due to a decrease in the temporary rigidity.
Several ways to improve the rigidity:
Practical advice:
1. By boiling. The water is boiled as described above, but the bottom layer is used.
2. By mixing with more hard water.
3. Adding small pieces of limestone, chalk, marble chips, shells, colored glass.
4. By adding magnesium chloride and calcium chloride to the water, soda.
5. Adding to the aquarium shells of rapans, coral crumbs (you need to boil for a long time)
ELECTRICAL CONDUCTIVITY (microSiemens)
Going on a trip to the tropics, every professional will certainly take an electronic tester to measure the electrical conductivity: there are very few water in the world that are clean enough that they do not conduct electricity. But in South America, they are still quite often! For water to conduct electricity, it must contain ions (electrically charged particles). The electrical conductivity of water also determines the "osmotic ratio" (the electrolyte content) in the aquarium water. Osmotic pressure is critical for biological indicators of spawning water. In most cases, the reproduction of fish is possible only when the artificial conditions are as close to natural as possible. In the tropics, in the homeland of aquarium fish, water is often very mild and poor in mineral salts. As already mentioned above about the rigidity of water, in the Amazon basin there are often such extreme water indicators that you just wonder how fish can exist there, for example, at a pH of 4.5-4.9. As is known, in this water lives, in particular, the red neon (Рaracheirodon axelrodi) and until the last few years this small pearl of aquariumism could not be made to breed in our conditions, as well as some sorts of outbreaks from Southeast Asia. Recent achievements in this area are primarily related to scientific conclusions about the relationship between the electrical conductivity of aquarium water and osmotic pressure. The electrical conductivity of water is measured using a small pocket device: a transistor tester. This device is relatively inexpensive and gives the interested aquarist an accurate indication.
 |
Electronic conductivity tester (in this case with a digital indicator) is quite small in size, and therefore convenient. They are designed specifically for aquarists by different companies: Tunze, Dupla, Stein, Bischof and others. |
Determine the electrical conductivity should be at 20 ° C. If the measurement should be carried out in the open air, where it is not possible to bring water to this temperature, then it is necessary to set the actual temperature. Then the result will be, for example, NS26. The water temperature has a decisive influence on the result. The values from 25 to 140 nS are ideal for reproduction of fish. But it should be stressed once again that distilled water, although it can have 0 ° stiffness, but almost never 0 nS electrical conductivity. Practice shows that the degrees of conductivity of distilled water are always high enough. If you colonize fish in a tank, where water has other indicators, problems may arise. Therefore, if the conductivity is changed too abruptly, aquarists from the precaution are gradually replacing the producers. In this way, they can be helped to adapt to new life conditions. According to their nature, fish originating from very poor in minerals waters, for the breeding of young animals need the same water, even if many of their generations were kept in water more rigid, and therefore rich in minerals. The reason for this is the structure of the fish egg. Eggs, like sperm cells, consist of cells enclosed in a very thin shell, the so-called membrane. Cells contain, in particular, water, and there are mineral substances in it. The very eggs are also surrounded by water, and mineral salts are dissolved in it again. Thus, here there are two elements colliding with each other, separated only by the above-mentioned thin membrane and seeming identical, but in fact often not being such.
 |
 |
 |
| An example that should clarify the effect of osmosis on caviar: in trying to adapt to the concentration of internal and external solutions, the egg may swell or contract. Both that, and another destroys the ability to develop, makes the eggs unsuitable for reproduction. |
We have already mentioned that the determination of the electrical conductivity and the result of measurements depend on temperature. As soon as the temperature rises by at least 1 ° C, the measured value also increases by approximately 2%. Most often it is recalculated with respect to 20 ° C. How this is done, you can understand from the table.
| Water temperature in ° C when measuring
|
Temperature coefficient in relation to 20 ° С
|
| 15
|
1,132
|
| 16
|
1.095
|
| 17th
|
1.071
|
| 18
|
1.046
|
| 19
|
1.023
|
| 20
|
1,000
|
| 21
|
0,979
|
| 22
|
0.958
|
| 23
|
0.937
|
| 24
|
0.919
|
| 25
|
0.901
|
| 26th
|
0.840
|
| 27th
|
0,810
|
| 28
|
0.790
|
| 29
|
0.770
|
| thirty
|
0,750
|
Now consider another indicator of water:
SALT COMPOSITION AND REGULATION OF OSMOTIC PRESSURE
One of the main problems of all aquatic inhabitants, regardless of the complexity of their organization, is the regulation of osmotic pressure. Osmotic pressure develops as a result of the diffusion of water molecules through the semipermeable membrane of living cells. It is based on the property of membranes to selectively pass molecules of certain substances and to trap molecules of others.
With different concentrations of salts on both sides of the membrane, for example inside the cell and outside it, the diffusion of water molecules into the region of higher salt concentration increases. Since the concentration of soluble substances and proteins in the cell is greater than in fresh water, freshwater organisms are forced to do a tremendous job of removing the excess water that penetrates into their body.
One of the evolutionary devices aimed at limiting the penetration of water into the tissues of freshwater fish is the concentration of salts in their blood and tissue fluids that is lower than in marine fish. In addition, freshwater fish have developed kidneys, which ensure the removal of excess water from the body. The urine of these animals contains less salts than blood and tissue fluids.
It is often enough that the function of salts dissolved in water reduces to their indirect effect on fish through food chains. The basic mineral components of the medium are phosphoric and nitrogen salts. This is due to the role that molecules play in living cells containing atoms of phosphorus and nitrogen. Less important are potassium and calcium, as well as sulfur and magnesium. In the latter, in addition to animals, plants are in great need, as this element is absolutely necessary for the biosynthesis of chlorophyll. The effect of salts is enhanced with an increase in temperature, which is associated with an increase in the intensity of metabolic processes. In smaller quantities, but not least, so-called trace elements - cobalt, manganese, copper, zinc, boron, iodine, silicon and some others - are needed.
On fish, the excessive content of calcium and iron salts in water is painfully reflected. Even an insignificant admixture of the latter causes eye diseases in adult fish, and in fry the defeat of gills accompanied by mass death.
Many substances, dissolved in water, can be attributed to poisonous or harmful. These are hydrogen sulphide, carbon dioxide and ammonia - natural products formed in reservoirs, as well as heavy metal compounds, inorganic and organic products of effluents from industrial enterprises. The last group of poisons in an aquarium does not occur.
The quantity of salts in fresh water is negligible (does not exceed a tenth of a gram per liter) and varies in a freshwater aquarium, depending on the soil, the content of the decomposition products, the volatility of water. These changes are small, and fish and plants adapt easily to them. In some cases, aquarists deliberately slightly increase the salinity by adding table salt.
Now a little about light, i.e. About the illumination of water.
WATER LIGHTING
Next, I would like to dwell a little on such a parameter as the water saturation, but very briefly, because You can learn more about this from the section: Lighting in an aquarium.
Here we just wanted to touch on this issue in general terms.
So:
The existence of life on Earth is possible due to the energy of the Sun. Light is one of the most volatile and at the same time the most regular in its impact environment factor. It is a necessary condition for the existence of plant organisms, as well as for animals that eat them. Pisces light is necessary for orientation in space, finding food and timely reaction to the approach of a predator.
For animals living in water, the main source of light is solar radiation, which comes from the atmosphere to the surface layers of water. The amount of penetrating light is determined not only by the time of day or by the transparency of the atmosphere, but also by the state of the water surface: a smooth surface reflects much less sunlight. A certain part of the solar radiation is absorbed by water, and the blue rays are absorbed less than others; Red rays are absorbed much more strongly.
The main organ of perception of light in fish is the eye. Most fish distinguish color. The spectrum of colors perceived by fish is largely determined by the ecological characteristics of the habitats. Naturally, the inhabitants of the upper layers of the water, like the fish of shallow water and coastal zones, distinguish much more flowers than deep-sea fish. At dusk, fishes are only perceived by short-wave rays.
The coloration of the body of most fish is closely related to the characteristics of the lighting, which in turn depend on the conditions of habitats and animal biology. It is changeable and can undergo significant changes in the process of individual development. Often it changes within a day.
A significant role is played by illumination in the development of fish, regulating puberty and the features of the sexual cycle.
For the normal life of fish and plants in the aquarium requires a different need for lighting. In practice, the duration of a light day is required from 8 to 10 hours per day.
Light is natural, mixed and artificial. If the aquarium is not far from the window, then it will be fully provided with natural light.
Mixed lighting is usually used in autumn and winter, as well as when seeking decorative effect or when water plants are mainly grown.
Artificial lighting depends on the strength of the lighting from the window and the design of the aquarium. For the maintenance, and often for the breeding of most different species of fish and plants, it does not really matter if the aquarium is illuminated by natural or artificial light. However, the advantage of the latter is that its power is easily regulated. The illumination of the aquarium by sunlight has its drawbacks, the main one of which is the difficulty in controlling the intensity and duration of natural light. In addition, when installing the aquarium at the window of the fish look inexpressive.
The intensity of illumination in an aquarium should correspond to the intensity of illumination inherent in fish and plants in natural reservoirs. It is regulated by experience. In this case, it is necessary to take into account the volume of the aquarium and the number of living organisms that inhabit it.
Now a little about the water temperature.
WATER TEMPERATURE
The range of temperatures known to us is very large - from many thousands of degrees on the Sun and other stars to near zero absolute cold of outer space. Under the conditions of the Earth's biosphere, the difference between the maximum and minimum temperatures is much lower and does not even reach several hundred degrees. In a much smaller interval, measured by several tens of degrees, most of the living beings known to us are able to grow, develop and reproduce. For some organisms, this range is even narrower.
In the water environment, the temperature fluctuations are much less pronounced than in the atmosphere, which is due to the high specific heat of water. The upper limit for the vast majority of fish species is +40 degrees Celsius, and the lower limit is close to the freezing point of water, and this temperature is tolerated by most species.
The temperature of the fish affects the body in two ways. Primarily this direct effect, especially essential for fish, as animals with unstable body temperature. In most fish, body temperature is only 0.5-1 degree C above the temperature of the aquatic environment. If we consider that most metabolic processes in the body are controlled by enzymes whose activity is very dependent on temperature, then the relationship between the temperature of the aquatic environment and the intensity of metabolism will become quite obvious.
For each fish species, there is a certain upper and lower limit of the water temperature; If this limit is violated, the fish dies. Of course, there are cases when, with a significant drop in the temperature of the water, the fish do not die, but simply become sluggish, but without harmful consequences (for example, loss of ability to reproduce, various diseases).
With increasing temperature, the metabolic processes in the fish body are increased, which causes an increase in oxygen consumption. It is known, however, that with increasing temperature the solubility in water of gases and, in particular, oxygen, decreases. In this way, an indirect effect of the increase in temperature on fish is realized, when, contrary to the increasing needs of the body, oxygen supply decreases, and animals eventually die from suffocation. It should be noted, however, that the increase in metabolism (in particular, the rate of digestion of food) is observed only in the region of optimum temperatures for a given fish species. On exceeding a certain threshold, various physiological and biochemical protective mechanisms are included, due to which, for example, cold-water fish respond to high temperatures by reducing the intensity of nutrition and a sharp decrease in activity.
If you transplant fish from warm water into a cold one, they will experience a shock state, which is apparent in the fact that the fish swim slowly, languidly moving fins and gills, or are immovable lying on the bottom. In the end they perish. If you transplant fishes from cold water to warm water, they, on the contrary, rush through the aquarium, try to jump out of the water.
Practical advice:
To avoid this, transplanting fish is necessary only when the temperature in both vessels is the same or not exceed 2 degrees C when transplanting from warmer water to a colder one and 4 degrees C from cooler water to a warmer one. When transplanting fry, this difference should be two times smaller. It is generally not advisable to transplant tropical fish from warmer water to a colder one.
The water temperature largely regulates such important aspects of the life cycle of fish as the maturation of sexual products and the development of fertilized eggs. Acceleration of ripening of eggs or sperm is associated with a general increase in the intensity of metabolism. In addition, these processes are influenced by the food supply of the producers themselves and the young. This, in particular, explains the spawning of many tropical fish species, the juveniles of which almost all year round can find the necessary food.
In nature, fluctuations in water temperature are observed, caused by its daytime heating and night cooling. The amplitude of these oscillations can sometimes reach 10 degrees C or more. If in a natural aquatic environment the fish can rise or fall in layers with the optimum temperature for them, then in the aquarium they have such an opportunity. In this regard, for most fish, the amplitude between the maximum and minimum temperature should not exceed 2-3 degrees C, and during the spawning period - 1 degree C. Maintaining the temperature for a particular species or group of species is mandatory. How to achieve a constant temperature you will learn from the section: Heating the aquarium.
Practical advice:
Despite the fact that all fish tolerate a short stay in the water, the temperature of which has turned out to be below optimal, it should not be, after discovering its omission, strive for a rapid temperature rise - it is enough to eliminate the malfunction, contributing to a smooth temperature setting at the required level.
And one more short advice in conclusion: When acquiring fish, first of all find out at what temperature they lived in the same place. Know this is necessary in order to gradually accustom them to the temperature of water in these conditions. You should also act by purchasing aquatic plants.
Well, in conclusion of the topic of properties and parameters of water, it is necessary to tell a little about the content of oxygen, carbon dioxide, hydrogen sulfide and nitrogen in water.
OXYGEN, CARBONATE GAS, HYDROGEN SULFUR, NITROGEN IN WATER
The absorption of oxygen by animals and the removal of carbon dioxide are as necessary as digestion and assimilation of food, and is the basis of all life processes. The need for oxygen is determined by the energy expenditure of the body on movement, the work of internal organs, and the needs of each cell in the body. It is necessary to distinguish between the physiological processes of oxygen and carbon dioxide exchange between the body and the external environment (gas exchange) and the biochemical processes of using oxygen and the formation of carbon dioxide in cells (tissue or cellular respiration). Gas exchange is a very important process for the body, the effectiveness of which ensures, in the final analysis, its survival.
Both oxygen and carbon dioxide (CO3, another name - carbon dioxide) are gaseous substances, it is in this form that they are absorbed or excreted by terrestrial animals. Their ratio in the atmospheric air averages 700: 1, which creates favorable opportunities for breathing. In water, this ratio is completely different. Due to the limited solubility, the maximum oxygen content in the water is approximately 20 times less than in the air. Carbon dioxide, unlike oxygen, can not only dissolve, but also react with water chemically, forming carbonic acid. The physical process of dissolving carbon dioxide takes place mainly in an acid medium. In a neutral and especially alkaline environment, a significant portion of carbon dioxide enters into chemical reactions with the salts contained in the water.
The worst (in comparison with terrestrial animals) supply of aquatic animals with oxygen is compensated to a certain extent by the ease of recycling carbon dioxide due to its chemical binding. Although gas exchange is facilitated in this way, in the specific conditions of the aquatic environment the main problem remains - the availability of oxygen. This caused the emergence of a variety of adaptations of organisms.
It is known, for example, that at different stages of their individual development, animals suffer a different oxygen deficiency. Thus, in fish that live and breed in lakes with a low oxygen content, small caviar is often found. This leads to an increase in the ratio of the surface of the egg to its volume, which facilitates gas exchange. Caviar of other fish has adaptations that ensure its development on an oxygen-rich surface or in the water column. Seemingly incomprehensible, the presence in many fish of the flowing waters of the bottom caviar is associated with a much better supply of their oxygen in comparison with the near-bottom zones of standing waters. Thus, for most fish (as for other aquatic animals), oxygen very often is a factor limiting their development and dispersal.
As already mentioned, carbon dioxide is one of the final products of the metabolism of living cells. Gas exchange of hydrobionts, as well as dissolved in water carbon dioxide of air - the main sources of carbon dioxide in water. Dissolution of CO 3 is accompanied by the formation and dissociation of carbonic acid and promotes acidification of the aquatic environment. In turn, the absorption of CO2 by plants in the process of photosynthesis reduces the active reaction of the aquatic environment (PH), which, with a significant development of phytoplankton in the flowering period, is shifted to the alkaline side. In this case, plants not only completely consume dissolved CO2, but also contribute to the transition of hydrocarbons to carbonates. Plants, unlike animals suffering from an increase in the content of СО3 in water, respond to this by intensifying photosynthesis.
Carbon dioxide, or carbon dioxide, when dissolved in water forms a weak acid (in the literature it is often also called carbonic acid). But the aquarist should not confuse different terms when they are written in the form of chemical formulas: Coal - C (from Carbonum, coal). Carbon monoxide, carbon monoxide - CO. Carbon dioxide, carbon dioxide - CO (gas without odor and color, is also contained in plant dressings). Carbonic acid - H 2 CO 3 (water-soluble carbon dioxide, weak acid).
The first conclusion: carbon dioxide makes the water acidic. This is the very reason why at the waterworks near the water before putting it into the consumer's network, reduce acidity. The acid is aggressive and could affect the piping system. Any natural water contains carbonic acid in various amounts, in a dissolved or bound form. The carbonic acid binds to the compounds of calcium and magnesium, in other words: that in the water there is calcium, there must be some amount of free carbon dioxide.
If the carbon dioxide content is excessive, it is called free or dissolved. The higher the proportion of calcium bicarbonate in water, the higher the proportion of bound carbon dioxide. Under the fertilization of CO 2 in aquaristics is meant the feeding of aquarium plants with carbon dioxide using a diffuser. To assimilate carbon dioxide, plants need a lot of light. Only through the light can the process of assimilation begin, and the thorough absorption of CO 2 plant leaves proves that they release tiny bubbles of oxygen. If the supply of carbon dioxide in the aquarium water is excessive, this will affect the decrease in the pH value. Too strong carbon dioxide intake interferes with the free breathing of fish and brings harm: the fish hang right under the surface of the water and try to pass through their gills oxygen-rich water.
In the damaged aquarium environment on the upper side of the leaves of plants sometimes there are calcareous deposits. This phenomenon, called "biogenic lime deposition" or "bicarbonate assimilation," is manifested even more strongly by the higher carbonate hardness of water with simultaneous powerful illumination, in which case, because of the lack of carbon dioxide, the process proceeds in the reverse order: since there is no free or dissolved carbon dioxide , The plants absorb calcium bicarbonate from the underside of the sheet, dissolve the bound carbon dioxide inside the sheet and release calcium hydroxide-Ca (OH) 2 from the upper side.The carbonate hardness of the water decreases, and the pH value increases.The leaves show a grayish plaque, and their surface To the touch becomes quite stiff (as if sprinkled with powder).
Many aquarists know that in soft water plants develop poorly. First of all, this is due to the fact that the absence of lime is the absence of a shock absorber for carbon dioxide. On the other hand, when using the so-called CO 2 fertilizer, a small addition of carbon dioxide is sufficient to feed the plants abundantly. At night, the process of assimilation is suspended, and therefore the fertilization of CO2 plants must also be stopped.
The source of oxygen for aquarium fish is aquatic plants and atmospheric air. If the surface of the aquarium is large enough, and the level of constantly stirred water is low, in this case a significant amount of oxygen comes from the air. This type of aquarium is usually used as a spawning ground; They should not contain a large number of fish. In an ordinary aquarium, where the water surface is small, and the level is high, atmospheric oxygen enters the water a little.
To saturate the water with oxygen, there are two ways: mechanical and biological. The first is aeration of the aquarium with the help of a compressor. About this method, we will describe in detail in the section: Aeration and filtration.
The air coming from the sprayer from the lower layers of water in the aquarium comes to the surface in the form of bubbles, while the water comes into contact with air and is enriched with oxygen. The second way is the release of oxygen by aquatic plants.
If there are no aquatic plants in the aquarium, the fish do not have enough oxygen; In this case, they keep on the surface of the water at an angle of 45 degrees and strenuously grab the air with their mouths. Such oxygen starvation often leads to disease and death of fish. If there is an excess of plants in the aquarium, it should be well illuminated so that the process of photosynthesis and oxygen evolution takes place, otherwise fish can also die from suffocation.
Oxygen should be dissolved in any aquarium in as large a quantity as possible. Oxygen is a gas whose solubility in water depends on temperature: the warmer the water, the faster oxygen escapes. It can not be considered only as an element necessary for the life of fish: purification of water from poisons also depends on oxygen, because the decomposition of poisonous substances is provided primarily by oxygen-dependent bacteria. Water can absorb oxygen everywhere, but in natural waters (rivers, lakes, ponds) this happens almost exclusively on the surface. The water of wells and sources of oxygen is therefore poor.
If the aquarium water is actively enriched with oxygen due to the supply of external air, then it can displace the existing carbon dioxide. In the literature on aquarium, the term "oxygen saturation" is often used.
Yes, it is really possible to achieve not only saturation, but also supersaturation of water with oxygen, if by the assimilation of plants it accumulates in excess. The absorption of oxygen is determined by the temperature of the water. The colder the water (above the freezing point), the more oxygen it can take. This applies to other gases, for example, carbon dioxide, although on a different scale.
In the following practice, you can use the following table:
| Water temperature in ° C
|
Oxygen saturation in mg / l
|
| 0
|
14.2
|
| 6th
|
12.1
|
| 12
|
10.0
|
| 18
|
9.2
|
| 24
|
8.2
|
| 26th
|
8.0
|
| 28
|
7.7
|
| thirty
|
7.5
|
| 32
|
7.3
|
| 34
|
7.1
|
| 40
|
6.6
|
What does this table tell the aquarist? If the water temperature exceeds the norm for the reproduction of fish or because of the need for therapy (treatment of the disease), the oxygen supply should be increased accordingly. An experienced aquarist learns from the frequency of breathing of his fish when the moment has come, True, if the fish are inhaled more quickly than usual, do not draw conclusions solely about the lack of oxygen. This may be attributed to other things, for example, poisoning or gill parasites (see section: Fish Diseases ).
The oxygen content of the water is measured using indicators that can be purchased at specialized stores.
Practical advice:
To avoid these troubles, it is possible, if an optimal light regime is established, water is aeration with the help of a compressor, weekly replace 1/5 of the water from the bottom to fresh, siphonize the ground.
In high aquariums without artificial aeration of water in the bottom layers, there is a lack of oxygen. In this case, the remains of food not eaten by the fish, which have fallen to the bottom, are not oxidized, but decay. This happens in the event that the aquarist gives the fish an excessive amount of live and dry food. In the process of rotting, hydrogen sulphide is released, which can lead to the death of fish. The same situation occurs when the soil in the aquarium consists of very fine sand, which prevents the penetration of oxygen into the soil. Signs of the presence of hydrogen sulphide are the darkening of the upper layer of the soil and the smell of rotten eggs from the bottom of the aquarium, from which water is drained. In the case of the appearance of hydrogen sulfide in water, it is necessary to regularly aerify the aquarium with air; Also, sand should be replaced with a larger one.
Nitrogen is poorly soluble in water and therefore is not dangerous for fish. However, in the case of excessive aeration of the aquarium, under excessive pressure, nitrogen bubbles can accumulate and lead to clogging of the blood vessels of fish. More dangerous for them are nitrogen compounds, which are products of decomposition of organic substances, such as fodder residues, excrement, and the like. These compounds include ammonia (NH3) and ammonium (NH4), which can penetrate into fish tissues. The ratio of these substances depends on the acidity of the water. At high pH, ammonia is more toxic than ammonium; With a low oxygen content in the water, the toxicity of nitrogen compounds increases. Ammonium content in the amount of 0.2 mg / l is permissible, and the same amount of ammonia leads to the death of fish.
Practical advice:
When changing the water in the aquarium, do not top up a large amount of water at once, because in the event of an increase in the pH of the water, a reaction may occur, as a result of which ammonium can pass into ammonia.
Under the action of nitrifying bacteria, ammonia and ammonium are oxidized first to nitrogen (HNO2), and then to nitrogen (HNO3) acid. Nitrous acid salts - nitrites are unstable, but even a small number of them is dangerous for fish, since it negatively affects blood hemoglobin, tissue and vascular system of fish; Salts of nitric acid - nitrates are less toxic. In the absence of plants in the aquarium, as well as overpopulation of fish in the aquarium, excessive accumulation of nitrates occurs, which in turn leads to the conversion of nitrates to nitrites.
Practical advice:
To avoid such trouble, you should change 10-15% of water every week, and avoid overpopulation of the aquarium.
|
|









Comments
When commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.