Interesting facts about Zelenka
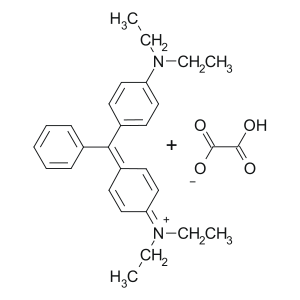
Brilliant green (tetraethyl-4,4-diaminotriphenylmethane oxalate) (Viridis nitens) is a synthetic aniline dye of the triphenylmethane series.
Technical names - main green 1; 42040; The main bright green; Malachite green G.
Antiseptic; Apply in the form of aqueous or alcoholic solutions for lubricating the skin in its diseases and injuries.
Not to be confused with the closely related substance - malachite green (tetramethyl-4,4-diaminotriphenylmethane).
Interesting facts about Zelenka

For the born in the USSR zelenka - a symbol of ineradicable will and confident prosperity. Zelenka was (and remains anywhere) a universal means of combating a variety of diseases.
However, not everyone knows what caused such a popularity of this medical product, why it is "brilliant" and why Western medicine refuses to use such a magical doctor.
1. The history of its origin goes back to the 19th century and the weaving industry. A young London chemist, William Perkin, was at the time working on a drug for malaria. The result of one of the experiments was a substance colored in an unnaturally bright purple color. It was impossible to wash this color from clothes. The resourceful father, from an unexpected experiment, "squeezed out" the practical benefits and opened a plant for the production of aniline dyes.
2. Doctors used dyes for their own purposes. They stained the preparations to better visualize the life of microorganisms under the microscope. Suddenly, physicians discovered that one of the dyes, namely the brilliant green, destroys germs. Since then, this antiseptic drug has confidently entered the medical practice. Today, many effective antiseptics have been invented, but the green does not hurry to give way to analogs.
3. Zelenka in dry form - this is a lump of golden green color. The Latin name of this substance is viridis nitentis, in literal translation - green shining. One of the scientists, translating the term into French, used the word brillant, which does not contradict the meaning (brillant in one of its meanings is brilliant). Confusion occurred when translating from French into Russian. Brillant was used in the sense of - diamond. Among all the dyes, only diamond green is distinguished by such a pompous name.
4. It is worth noting that for today, green is used only in Russia and some CIS countries. What caused this dislike from the rest of the world? European scientists find it difficult to give a clear answer. They argue their dislike with the following provisions:
- The mechanism of action of green is not sufficiently studied. Western medicine is not going to apply unexplored scrupulously. How to spend money and time on experiments with greenery
- Aesthetic side. Well, I do not like the extremely civilized part of the planet this way of "greening" the patient. Why spoil the appearance, when around such an abundance of inconspicuous antiseptics?
By the way, the widespread view that zelenok - a strong carcinogen, in fact, can only be a myth. Appropriate studies on this subject have not been conducted.
5. Did you know that:
- For a white rat, a dose of 0.05 g / kg is a lethal dose,
- Green in the industry is used for painting (cotton, silk, paper, etc.)
- Chemical formula green - C27H33N2 * HC2O4 * H2O,
- In the days of Stalin's repressions on the body of the executed prisoner's number was deduced precisely "brilliant green".
History and etymology
Brilliant green was first obtained in 1879 in Germany. The antiseptic properties of this substance were learned only in the next century. In the middle of the twentieth century, brilliant green, durable and cheap in production, was widely used in the Soviet Union.
In Russian, the name of this dye came from the French language. In dry form, the diamond green represents a golden-green lump, in Latin viridis nitens, literally "green shiny". When translated into French, the word brillant was used - in French "brilliant", that the Russian translator mechanically translated as "brilliant".
In addition to Russia and several countries in the post-Soviet space, the brilliant green in medicine is nowhere used anymore, although, for example, in Europe, it is on the list of permitted medications. Possible reasons can be three. First, in the Western countries the doctrine of evidence-based medicine has been adopted, and the molecular mechanism of action of this (and other) dyes is still unknown. Secondly, it is still not known exactly whether the diamond green has carcinogenic properties. Thirdly, when used for a medicinal product, the aesthetic side is also important, why in western medicine are taken into account and changes in the appearance of the patient when using the drug.
In the post-Soviet space a solution of brilliant green is widely known under the colloquial name zelenka. In the Soviet and Russian criminal environment, there is the expression "smear (someone) forehead greens," originally meant execution (death penalty), and later - and just murder (with the help of firearms). This expression arose in the days of Stalin's repression, when a prisoner who was shot or died was written with a green prisoner on his hip. Therefore, initially there was an expression: "to spread a green leg", but later they started talking about the "forehead", although the forehead did not have anything to do with the writing of the greens.
Properties
In Russia, the quality of the Brilliant green dye is regulated by TU 6-09-4278-88. The quality of the drug Brilliant green is regulated by the regulatory documentation (ND) of the producers of this antiseptic.
Physical:
- Greenish-golden lumps or golden-green powder.
- It is soluble in water (1:50) and ethanol, soluble in chloroform.
- Solutions in water and ethanol have an intense green color.
- The maximum of the light absorption curve at 625 ± 0.5 nm.
Chemical:
- When the solution of brilliant green concentrated hydrochloric acid is added to 0.2%, an orange color appears, and when a solution of NaOH is added, a pale green sediment of the base falls out (these reactions are used to establish the authenticity).
- Incompatible with disinfectant drugs containing active iodine, chlorine, alkali (including ammonia solution).
Receiving
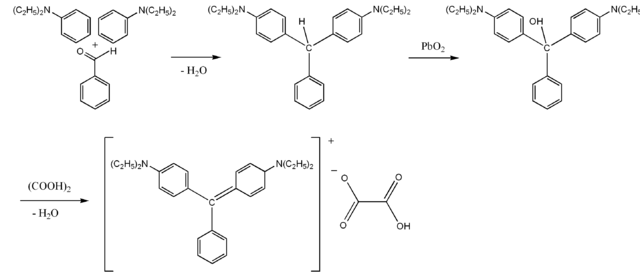
Brilliant green is obtained only by synthetic means. The synthesis is reduced to the condensation of diethylaniline with benzaldehyde; The resulting 4,4'-bis-diethylaminotriphenylmethane is oxidized with lead (IV) or manganese (VII) oxide; The carbinol base thus formed interacts with oxalic acid to form a brilliant green.
Application
Produced in the form of salts of bis (para-diethylamino) -triphenylanhydrocarbinol with various anions:
- Oxalate (CAS 23664-66-6) - the most widely used in medicine.
- Sulfate (CAS 633-03-4) - used for photometric determination of certain chemical elements and as a pH indicator.
- Base (CAS 630-98-8).
In medicine
On the territory of the former USSR, a solution of brilliant green is used as an antiseptic drug (in the US and the European Union, it is allowed as an antiseptic, but not applied).
As a medicine, 1% or 2% alcoholic solution of oxalate is used (on 57% ethanol, less often - in the form of an aqueous solution, from 0.1% to 2%, according to the recommended dosage), also available as a pencil.
It is indicated for use in decontamination of fresh postoperative and post-traumatic scars, umbilical cord of newborns, abrasions, cuts, other skin integrity disorders, in the treatment of pyoinflammatory skin processes - gordoleum (barley), meibomite, blepharitis, pyoderma, furunculosis, carbuncle, Staphylococcal infection. It is applied externally, the drug is applied to the damaged surface, capturing surrounding healthy tissues.
It is a highly active and high-speed antiseptic (active against Gram-positive bacteria), it also has a fungicidal effect against some pathogenic fungi. Gram-negative bacteria are practically not affected.
In the aquatic environment it is harmful to the culture of Staphylococcus aureus (Staphylococcus aureus) in a concentration of 1:10 000 000, its phenolic coefficient is 40 000. High sensitivity to diamond green is detected by diphtheria bacillus (Corynebacterium diphtheriae).
In the presence of organic substances, antimicrobial activity decreases: when the activity of this dye is evaluated in a medium containing 10% of blood serum, the phenol coefficient is 120 (0.3% of the value in the aqueous medium).
Also used in veterinary medicine.
In medical microbiology
In bacteriology and histology it is used for staining of cellular media.
It is also used for the preparation of agar medium with a brilliant green, intended for the re-culture and identification of bacteria of the genus Salmonella.
In industry
Brilliant green is used as a dye for cotton and silk, paper, wood (the colors are not resistant to light and wet treatments), is used for making fanal varnishes.
In agriculture
It is part of the Zar-2 drug used to limit the growth of strawberry and strawberry antennae (composition: chlorocholine chloride - 40%, acetic acid - 6.0%, brilliant green - 0.1%, drinking water - up to 100.0% ).
In Chemistry
In analytical chemistry, Brilliant green is used in the form of sulfate for the photometric determination of B, V, Sb, Re, Au, Ta, Tl, Hg, Zn, included in some anions, for spectrophotometric determination of iodine.
Toxicological chemistry and forensic toxicology is used as a quality reagent for thallium salts.
It is also used as a pH indicator for microscopy; With a transition from green at pH 0.1 to yellow at pH 2.6.
In art
The writer N. V. Gornov has a fantastic story "The Brilliant Green".
Toxic effect
In working conditions, the workers cause inflammatory skin diseases. In medical use, allergic reactions (itching, urticaria) are possible. If you get into the mucous membrane of the eyes, there is burning, lacrimation, a burn is possible.
Absolutely lethal dose for white rats 0.05 g / kg.


Comments
When commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.