Radiation is not always terrible: everything you wanted to know about it

After the accident at the Fukushima nuclear power plant, another wave of panic radio phobia swept over the world.
In the Far East, iodine disappeared from the sale, and the manufacturers and sellers of dosimeters not only sold all the devices in stock, but also collected pre-orders for six months or a year ahead.
But is radiation so terrible? If you every time wince at this word, the article is written for you.
What is radiation? This is the name of various types of ionizing radiation, that is, one that is capable of detaching electrons from the atoms of matter. The three main types of ionizing radiation are usually designated Greek letters alpha, beta and gamma. Alpha radiation is the flux of helium-4 nuclei (practically all helium from balloons was once alpha radiation), beta is a flux of fast electrons (less often positrons), and gamma is a flux of high-energy photons. Another type of radiation is the neutron flux. Ionizing radiation (with the exception of X-ray) is the result of nuclear reactions, so neither mobile phones nor microwave ovens are its source.
Charged weapons
Of all the arts, for us the most important, as is known, is cinema, and from the types of radiation - gamma radiation. It has a very high penetrating power, and in theory no barrier is capable of protecting it completely. We are constantly exposed to gamma irradiation, it comes to us through the thickness of the atmosphere from outer space, breaks through the layer of soil and the walls of houses. The reverse side of this all-pervasiveness is a relatively weak destructive effect: from a large number of photons only a small part will transfer its energy to the body. Soft (low-energy) gamma radiation (and X-ray) mainly interacts with matter, knocking out electrons from it due to the photoelectric effect, hard - it is scattered by electrons, while the photon is not absorbed and retains an appreciable part of its energy, so that the probability of destruction of molecules in such Process is much less.
Beta radiation, in its effect, is close to gamma radiation - it also knocks electrons out of atoms. But with external irradiation it is completely absorbed by the skin and the tissues nearest to the skin, not reaching the internal organs. Nevertheless, this leads to the fact that the flow of fast electrons transfers significant energy to irradiated tissues, which can lead to radiation burns or provoke, for example, cataracts.
Alpha radiation carries significant energy and a great momentum, which allows it to knock out electrons from atoms and even atoms from molecules. Therefore, the "destruction" caused by it is much larger - it is believed that by transferring to the body 1 J of energy, alpha radiation will cause the same damage as 20 J in the case of gamma or beta radiation. Fortunately, the permeability of alpha particles is extremely small: they are absorbed by the uppermost layer of the skin. But when ingested, alpha-active isotopes are extremely dangerous: recall the infamous tea with alpha-active polonium-210, which was poisoned by Alexander Litvinenko.
Neutral danger
But the first place in the rating of danger is undoubtedly occupied by fast neutrons. The neutron does not have an electric charge and therefore interacts not with electrons, but with nuclei - only with "direct hit". The flux of fast neutrons can pass through the layer of matter on average from 2 to 10 cm without interacting with it. And in the case of heavy elements, colliding with the nucleus, the neutron only deviates to the side, almost without losing energy. And in the collision with the nucleus of hydrogen (proton), the neutron transfers to it about half of its energy, knocking out the proton from its place. It is this fast proton (or, to a lesser extent, the core of another light element) that causes ionization in matter, acting like alpha radiation. As a result, neutron radiation, like gamma quanta, easily penetrates the body, but it is almost completely absorbed, creating fast protons, causing great damage. In addition, neutrons are the very radiation that causes induced radioactivity in irradiated substances, that is, turns stable isotopes into radioactive substances. This is an extremely unpleasant effect: say, from vehicles after staying in the focus of a radiation accident, alpha, beta, and gamma-active dust can be washed away, but it is impossible to get rid of neutron activation - the body itself (this, by the way, The damaging effect of the neutron bomb, which activated the armor of tanks.
In nature, neutron radiation is very insignificant. In fact, the risk of being exposed to it exists only with nuclear bombing or a serious accident at a nuclear power plant, with the most part of the reactor core being melted and emitted into the environment (and even only in the first seconds).
Gas discharge counters
Radiation can be detected and measured using a variety of sensors. The simplest of them are ionization chambers, proportional counters and gas-discharge Geiger-Muller counters. They are a thin-walled metal tube with gas (or air), along the axis of which the wire-electrode is stretched. The voltage is applied between the body and the wire and the flowing current is measured. The principal difference between the sensors is only in the magnitude of the applied voltage: at low voltages we have an ionization chamber, at large - a gas-discharge counter, somewhere in the middle - a proportional counter.
Ionization chambers and proportional counters make it possible to determine the energy that each particle transmitted to the gas. The Geiger-Muller counter only counts the particles, but the readings from it are very easy to receive and process: the power of each pulse is sufficient to directly output it to a small speaker! An important problem of gas discharge counters is the dependence of the counting rate on the radiation energy at the same level of radiation. For its alignment, special filters are used, absorbing a part of soft gamma and all beta radiation. To measure the flux density of beta and alpha particles, such filters are made removable. In addition, to increase the sensitivity to beta and alpha radiation, "end counters" are used: it is a disk with a bottom as one electrode and a second spiral wire electrode. The cover of the end counters is made of a very thin (10-20 microns) mica plate, through which soft beta radiation and even alpha particles easily pass.
Semiconductors and scintillators
Instead of an ionization chamber, a semiconductor sensor can be used. The simplest example is a conventional diode, to which a blocking voltage is applied: when an ionizing particle hits the pn junction, it creates additional charge carriers, which lead to the appearance of a current pulse. To increase the sensitivity, so-called pin diodes are used, where between the layers of p and n semiconductors there is a relatively thick layer of undoped semiconductor. Such sensors are compact and allow measuring particle energy with high accuracy. But the volume of the sensitive region is small, and therefore the sensitivity is limited. In addition, they are much more expensive than gas discharge ones.
Another principle is the calculation and measurement of the brightness of flares that occur in certain substances when particles of ionizing radiation are absorbed. You can not see these flashes with the naked eye, but special highly sensitive devices - photoelectric multipliers - are capable of this. They even allow you to measure the change in brightness over time, which characterizes the energy loss of each individual particle. Sensors on this principle are called scintillator ones.
Shield from radiation
To protect against gamma radiation, heavy elements, such as lead, are most effective. The larger the number of the element in the periodic table, the stronger the photoelectric effect appears in it. The degree of protection depends on the energy of the radiation particles. Even lead weakens radiation from cesium-137 (662 keV) only twice for every 5 mm of its thickness. In the case of cobalt-60 (1173 and 1333 keV), for a two-fold attenuation it will take more than a centimeter of lead. Only for soft gamma radiation, such as cobalt-57 (122 keV), a thin layer of lead will be a serious protection: 1 mm will weaken it once every ten. So the anti-radiation costumes from films and computer games in reality protect only from soft gamma radiation.
Beta radiation is completely absorbed by the protection of a certain thickness. For example, beta-radiation of cesium-137 with a maximum energy of 514 keV (and an average of 174 keV) is completely absorbed by a layer of water 2 mm thick or just 0.6 mm aluminum. But lead for protection from beta radiation should not be used: too rapid inhibition of beta electrons leads to the formation of X-rays. To completely absorb the radiation of strontium-90, less than 1.5 mm of lead is needed, but in order to absorb the resulting X-ray radiation, another centimeter is required!
From external alpha-irradiation to protect the easiest: for this enough a sheet of paper. However, most of the alpha particles do not pass in the air and five centimeters, so protection may be required unless in the case of direct contact with a radioactive source. It is more important to protect yourself from getting alpha-active isotopes into the body, for which a mask respirator is used, and ideally - a sealed suit with an isolated breathing system.
Finally, the fastest neutrons are best protected by hydrogen-rich substances. For example, hydrocarbons, the best option - polyethylene. When testing collisions with hydrogen atoms, a neutron quickly loses energy, slows down and soon becomes unable to cause ionization. However, such neutrons can still activate, that is, convert to radioactive, many stable isotopes. Therefore, boron is often added to neutron shielding, which very strongly absorbs such slow (they are called thermal) neutrons. Alas, the thickness of polyethylene for reliable protection should be at least 10 cm. So it does not get much lighter than lead protection from gamma radiation.
Tablets from radiation
The human body consists of water more than three quarters, so the main effect of ionizing radiation is radiolysis (decomposition of water). The resulting free radicals cause an avalanche cascade of pathological reactions with the appearance of secondary "fragments." In addition, radiation damages chemical bonds in nucleic acid molecules, causing disintegration and depolymerization of DNA and RNA. The most important enzymes that have a sulfhydryl group SH (adenosine triphosphatase, succinoxidase, hexokinase, carboxylase, cholinesterase) are inactivated. In this case, the processes of biosynthesis and energy metabolism are violated, proteolytic enzymes are released from the destroyed organelles into the cytoplasm, self-digestion begins. In the risk group, primarily sexual cells, precursors of blood cells, cells of the gastrointestinal tract and lymphocytes, but neurons and muscle cells to ionizing radiation are quite stable.
Preparations able to protect from the effects of irradiation began to be actively developed in the middle of the 20th century. More or less effective and suitable for mass use were only some aminothiols, such as cystamine, cysteamine, aminoethylisothiouronium. In fact, they are donors - SH groups, substituting them for impact instead of "relatives".
Radiation around us
To face radiation face-to-face, accidents are not at all necessary. Radioactive substances are widely used in everyday life. Natural radioactivity is possessed by potassium - very important for all living element. Because of the small admixture of the isotope K-40 in natural potassium, "fonit" is the dietary salt and potassium fertilizers. Some old lenses used glass with an admixture of thorium oxide. The same element is added to some modern electrodes for argon welding. Until the middle of the twentieth century, devices with illumination based on radium were actively used (in our time radium was replaced by a less dangerous tritium). Some smoke detectors use an alpha emitter based on americium-241 or highly enriched plutonium-239 (yes, the same one from which nuclear bombs are made). But worry is not necessary - the health damage from all these sources is much less harm from anxiety about this.
Dose and power
When measuring and evaluating radiation, many concepts and units are used.
- - The exposure dose is proportional to the number of ions that create gamma and X-rays in a unit of air mass. It is customary to measure it in X-rays (P).
- - Absorbed dose - the amount of energy of radiation absorbed by a unit of mass of matter. Previously, it was measured in radars (rad), and now measured in greeks (Gy).
- - The equivalent dose additionally takes into account the difference in the destructive ability of different types of radiation. Previously, it was measured in "biological equivalents of rad" - bears (rem), now - in sieverts (Sv).
- - An effective dose takes into account the different sensitivity of the organs to radiation: thus, it is less dangerous to irradiate the hand than the back or chest. Previously measured in the same peers, now - in sievert.
The conversion of one measurement unit to another is not always correct, but it is generally accepted that the exposure dose of gamma radiation in 1 P will cause the body the same harm as an equivalent dose of 1/114 Sv. Translation is glad in gray and Baer in Sievert is very simple: 1 Gr = 100 rad, 1Sb = 100p. To transfer the absorbed dose to the equivalent dose, a radiation quality factor equal to 1 for gamma and beta radiation, 20 for alpha radiation and 10 for fast neutrons is used. For example, 1 Gy of fast neutrons = 10Sv = 1000 rem.
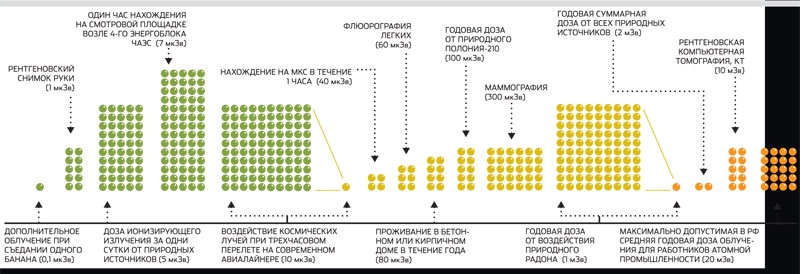
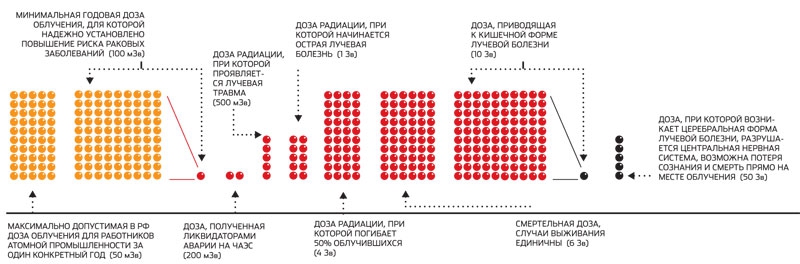
- - The natural equivalent dose rate (DER) of external irradiation is usually 0.06 - 0.10 μSv / h, but in some places it can be less than 0.02 μSv / h or more than 0.30 μSv / h. The level of more than 1.2 mkSv / h in Russia is officially considered dangerous, although in the cabin of the aircraft during the flight DER can repeatedly exceed this value. And the ISS crew is exposed to irradiation with a power of approximately 40 μSv / h.
Via popmech.ru



Comments
When commenting on, remember that the content and tone of your message can hurt the feelings of real people, show respect and tolerance to your interlocutors even if you do not share their opinion, your behavior in the conditions of freedom of expression and anonymity provided by the Internet, changes Not only virtual, but also the real world. All comments are hidden from the index, spam is controlled.