Платина

| 78 |
Платина
|
|
195,084
|
|
| 4f145d96s1 | |
Платина — благородный металл серебристо белого цвета. В таблице Менделеева этот химический элемент обозначается знаком Pt.
Платина (исп. Platina) — химический элемент 10-й группы (по устаревшей классификации — побочной подгруппы восьмой группы), 6-го периода периодической системы химических элементов Д. И. Менделеева, с атомным номером 78; блестящий благородный металл серебристо-белого цвета.
Название платине было дано испанскими конкистадорами, которые в середине XVI в. впервые познакомились в Южной Америке (на территории современной Колумбии) с новым металлом, внешне похожим на серебро (исп. plata). Слово буквально означает «маленькое серебро», «серебришко». Объясняется такое пренебрежительное название исключительной тугоплавкостью платины, которая не поддавалась переплавке, долгое время не находила применения и ценилась вдвое ниже, чем серебро.
История платины

Антонио де-Ульоа
Древний мир уже знал металлическую платину. При археологических раскопках в Египте в руинах древних Фив был найден художественной работы футляр, относимый специалистами к VII в. до н. э. В этой реликвии древнего мира находилось зерно богатой иридием платины.
В начале I в. н. э. промывальщики золотоносных песков в Испании и Португалии стали проявлять заметный интерес к полезному использованию «белого свинца», или «белого золота», как тогда называли платину. По свидетельству римского писателя Плиния Старшего (автора 37-томной книги «Естественная история»), «белый свинец» добывался из золотых россыпей Валиссии (Северо-западная Испания) и Лузитании (Португалия). Плиний рассказывает, что «белый свинец» собирался при промывке вместе с золотом на дно корзин и плавился отдельно.
Задолго до захвата Южной Америки испанскими и португальскими конкистадорами — платина добывалась культурным туземным народом — инками, не только владевшими секретом очистки и ковки этого драгоценного металла, но и умевшими искусно выделывать из него различные предметы и украшения.
Эпоха падения Римской империи знаменуется исчезновением из обихода ювелиров и торговцев драгоценностями из платины. Прошло много столетий, и только во второй половине XVIII в. платиной и ее физико-химическими свойствами начали интересоваться ученые.
В 1735 году испанский математик Антонио де-Ульоа, находясь в Экваториальной Колумбии, обратил внимание на частое нахождение совместно с золотом неизвестного ему металла, блеск которого несколько напоминал блеск серебра, но всеми прочими качествами более походившего на золото. Этот диковинный металл заинтересовал де-Ульоа, и он привез в Испанию образцы колумбийской платины.
В XVIII столетии, когда платина еще не имела промышленного применения, ее подмешивали к золоту и к золотым и серебряным изделиям. Об этой «порче» драгоценных металлов узнало испанское правительство. Опасаясь возможности массовой подделки золотой монеты, оно решило уничтожать всю платину, добываемую совместно с золотом в колониальных владениях королевства. В 1735 году был издан декрет, предписывавший уничтожать всю добывающуюся в Колумбии платину. Этот декрет действовал несколько десятилетий. Специальные чиновники в присутствии свидетелей периодически бросали наличные запасы платины в реку.
В конце XVIII в. испанские короли сами стали «портить» золотую монету, подмешивая к ней платину.
Техническое использование платины
Монета из платины
В 1752 году директор шведского монетного двора Шеффер объявил об открытии им нового химического элемента — платины. Спутники платины — палладий, иридий, родий, рутений и осмий — были открыты значительно позднее, в XIX веке. Шесть перечисленных химических элементов, стоящих в восьмой группе периодической системы Менделеева, составляют группу, именуемую платиновыми металлами. Все эти металлы обладают многими сходными физическими и химическими свойствами и встречаются в природе большей частью совместно.
На заре внедрения платины в технику ученые занимались ею большей частью из одного любопытства, но по мере углубленного изучения свойств платины она довольно быстро начала находить широкое применение, особенно в химической промышленности. Оказалось, что платина растворима только в царской водке, нерастворима в кислотах и постоянна при накаливании.
Вслед за появлением первых образцов химической посуды, изготовленной из платины, ее начали применять для изготовления перегонных аппаратов для серной кислоты. С этого момента стал резко увеличиваться рост обработки платины, так как ею стали пользоваться в производстве кислотоупорной и жароустойчивой лабораторной химической аппаратуры, инструментов и различных приборов (тиглей, колб, котлов, щипцов и т. д ).
В пирометрии используют исключительную устойчивость платины и ее сплавов к высоким температурам.
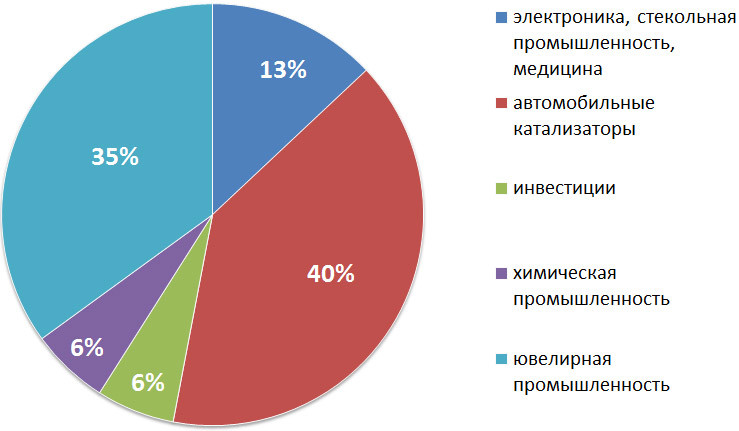
Ценные, а порой незаменимые свойства платины и палладия уже давно используются в каталитических процессах. Значительное количество платины расходуется на изготовление контакта для сернокислотных заводов, где она служит катализатором при окислении сернистого газа в серный ангидрид. Платина в виде сетки служит катализатором при окислении аммиака в аппаратах различных систем. Многочисленные органические синтезы также требуют применения платинового катализатора. Палладиевый катализатор применяется в производстве синтетического аммиака и при получении некоторых органических препаратов. В производстве синтетического аммиака по Габеру-Росеннолю применяется также осмий.
В электротехнике платиновые металлы, как правило, применяются в виде сплавов. Вот далеко не полный список деталей электроаппаратов, где используются платиновые сплавы: иглы для выжигания, приборы для электрических измерений, электроды (катоды и антикатоды для рентгеновских трубок), проволоки и ленты для сопротивлений электрических печей, контакты магнето (автомобили, двигатели внутреннего сгорания), контактные точки (телеграфия, телефония), наконечники громоотводов и т. д.
В электрохимии платина применяется при получении различных электролитических продуктов. Медицина и зубоврачевание являются одними из старейших потребителей платины. Отметим также применение платины для хирургии в виде наконечников приборов, служащих для прижигания, шприцев для впрыскивания и вливания и т. п.

Применение платины
Ювелирное искусство занимает ведущее положение как потребитель платины в виде сплавов. Платиновые оправы для драгоценных камней дают лучший блеск и более чистую воду, чем оправы из других благородных металлов.
Наконец, в виде солей платина и ее спутники требуются для фотографии, для изготовления лекарственных препаратов (соли родия и рутения) и для приготовления красок по фарфору (родий, иридий — черная краска, палладий — серебристая).
Платина используется и в военном доле, например для изготовления контактов, служащих для производства детонации при взрыве мин, и т. п.
Добыча платины

Как добывают платину
Первое место в мировой добыче платины принадлежит району Онтарио в Канаде. Здесь в 1856 году были открыты крупные месторождения медно-никелевых руд Сюдбери, в которых на ряду с золотом и серебром присутствует и платина.
До первой мировой войны канадская платина не привлекала к себе внимания, и практический интерес к ней возник только в 1919 году, когда вследствие гражданской войны на Урале добыча русской платины сильно упала, и мировой рынок стал ощущать большой недостаток в этом ценном металле. С 1919 года шламы медно-никелевого производства Сюдбери стали подвергать тщательной переработке с целью извлечения металлов платиновой группы, тем более что себестоимость попутной добычи платины и ее спутников очень низка.
Второе место в мире по добыче платины занимает Россия. Заметные количества платины добываются в Колумбии. Из других стран, производящих платину, можно указать Эфиопию и Конго. Добытая непосредственно из недр платина, а также платина, полученная из руд, подвергается специальной обработке или аффинажу. Аффинаж состоит из обычных процессов, применяемых в небольших масштабах в практике аналитических лабораторий — растворения, выпаривания, фильтрования, осаждения и т. д. В результате этих операций получается чистая платина и раздельно ее спутники.
Мировые запасы платины и Месторождения платины в Украине
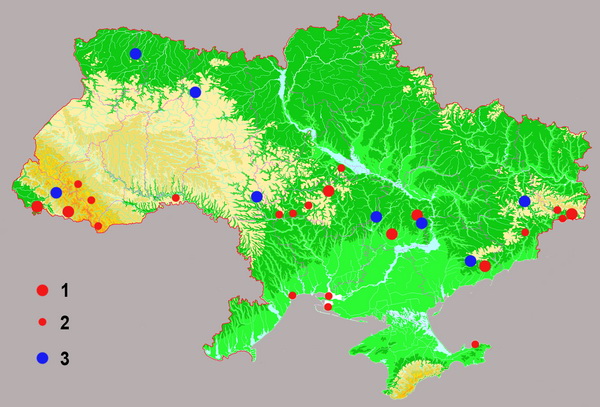
Схема расположения месторождений (1) и рудопроявлений (2) золота и проявлений платиноидов (3) на территории Украины.
Почти 90% мировых подтвержденных запасов металлов платиновой группы (МПГ: платина, палладий, иридий, родий, осмий, рутений) заключено в собственно платиноидных пластовых месторождениях позднемагматического генезиса. Мировые ресурсы платиноидов в недрах более 30 стран оцениваются в 120-140 тыс. тонн и большая их часть (75-85 тыс. тонн) находится в Бушвельдском массиве ЮАР. Объем мирового производства МПГ составляет примерно 370 т, из которых платины – 160 т, палладия – 180 т.
Изучение платиноидоносности геологических комплексов Украины имеет всего полувековую историю – начиная с выявления в 1951 году содержания платины (0,1-0,2 г/тонну) в гипербазитах реки Обиточной (Западное Приазовье). Спустя несколько лет присутствие платины и палладия было обнаружено в породах ряда других районов Украинского щита и в россыпях Днепровско-Донецкой впадины.
К числу перспективных геологических объектов на обнаружение платинового (и МПГ) оруденения в Украине относятся различные по составу породы Украинского щита, Карпат, Волыни, Криворожского и Донецкого бассейнов, Средних Приднепровья и Побужья (см. карту-схему).
Via wiki & alto-lab.ru & photoukraine.com
Created/Updated: 25.05.2018
 |
|